Impact of Xpert MTB/RIF testing on tuberculosis management and outcomes in hospitalized patients in Uganda
- PMID: 23139799
- PMCID: PMC3490868
- DOI: 10.1371/journal.pone.0048599
Impact of Xpert MTB/RIF testing on tuberculosis management and outcomes in hospitalized patients in Uganda
Abstract
Rationale: The clinical impact of Xpert MTB/RIF for tuberculosis (TB) diagnosis in high HIV-prevalence settings is unknown.
Objective: To determine the diagnostic accuracy and impact of Xpert MTB/RIF among high-risk TB suspects.
Methods: WE PROSPECTIVELY ENROLLED CONSECUTIVE, HOSPITALIZED, UGANDAN TB SUSPECTS IN TWO PHASES: baseline phase in which Xpert MTB/RIF results were not reported to clinicians and an implementation phase in which results were reported. We determined the diagnostic accuracy of Xpert MTB/RIF in reference to culture (solid and liquid) and compared patient outcomes by study phase.
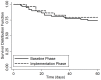
Results: 477 patients were included (baseline phase 287, implementation phase 190). Xpert MTB/RIF had high sensitivity (187/237, 79%, 95% CI: 73-84%) and specificity (190/199, 96%, 95% CI: 92-98%) for culture-positive TB overall, but sensitivity was lower (34/81, 42%, 95% CI: 31-54%) among smear-negative TB cases. Xpert MTB/RIF reduced median days-to-TB detection for all TB cases (1 [IQR 0-26] vs. 0 [IQR 0-1], p<0.001), and for smear-negative TB (35 [IQR 22-55] vs. 22 [IQR 0-33], p=0.001). However, median days-to-TB treatment was similar for all TB cases (1 [IQR 0-5] vs. 0 [IQR 0-2], p=0.06) and for smear-negative TB (7 [IQR 3-53] vs. 6 [IQR 1-61], p=0.78). Two-month mortality was also similar between study phases among 252 TB cases (17% vs. 14%, difference +3%, 95% CI: -21% to +27%, p=0.80), and among 87 smear-negative TB cases (28% vs. 22%, difference +6%, 95% CI: -34 to +46%, p=0.77).
Conclusions: Xpert MTB/RIF facilitated more accurate and earlier TB diagnosis, leading to a higher proportion of TB suspects with a confirmed TB diagnosis prior to hospital discharge in a high HIV/low MDR TB prevalence setting. However, our study did not detect a decrease in two-month mortality following implementation of Xpert MTB/RIF possibly because of insufficient powering, differences in empiric TB treatment rates, and disease severity between study phases.
Conflict of interest statement
Figures



References
-
- Golub JE, Bur S, Cronin WA, Gange S, Baruch N, et al. (2006) Delayed tuberculosis diagnosis and tuberculosis transmission. Int J Tuberc Lung Dis 10: 24–30. - PubMed
-
- Greenaway C, Menzies D, Fanning A, Grewal R, Yuan L, et al. (2002) Delay in diagnosis among hospitalized patients with active tuberculosis–predictors and outcomes. Am J Respir Crit Care Med 165: 927–933. - PubMed

