Phosphorylation controls the localization and activation of the lumenal carbonic anhydrase in Chlamydomonas reinhardtii
- PMID: 23139834
- PMCID: PMC3490910
- DOI: 10.1371/journal.pone.0049063
Phosphorylation controls the localization and activation of the lumenal carbonic anhydrase in Chlamydomonas reinhardtii
Abstract
Background: Cah3 is the only carbonic anhydrase (CA) isoform located in the thylakoid lumen of Chlamydomonas reinhardtii. Previous studies demonstrated its association with the donor side of the photosystem II (PSII) where it is required for the optimal function of the water oxidizing complex. However this enzyme has also been frequently proposed to perform a critical function in inorganic carbon acquisition and CO(2) fixation and all mutants lacking Cah3 exhibit very poor growth after transfer to low CO(2) conditions.
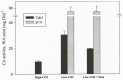
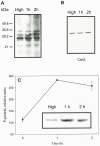
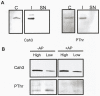
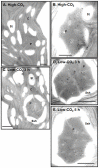
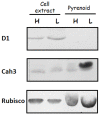
Results/conclusions: In the present work we demonstrate that after transfer to low CO(2), Cah3 is phosphorylated and that phosphorylation is correlated to changes in its localization and its increase in activity. When C. reinhardtii wild-type cells were acclimated to limiting CO(2) conditions, the Cah3 activity increased about 5-6 fold. Under these conditions, there were no detectable changes in the level of the Cah3 polypeptide. The increase in activity was specifically inhibited in the presence of Staurosporine, a protein kinase inhibitor, suggesting that the Cah3 protein was post-translationally regulated via phosphorylation. Immunoprecipitation and in vitro dephosphorylation experiments confirm this hypothesis. In vivo phosphorylation analysis of thylakoid polypeptides indicates that there was a 3-fold increase in the phosphorylation signal of the Cah3 polypeptide within the first two hours after transfer to low CO(2) conditions. The increase in the phosphorylation signal was correlated with changes in the intracellular localization of the Cah3 protein. Under high CO(2) conditions, the Cah3 protein was only associated with the donor side of PSII in the stroma thylakoids. In contrast, in cells grown at limiting CO(2) the protein was partly concentrated in the thylakoids crossing the pyrenoid, which did not contain PSII and were surrounded by Rubisco molecules.
Significance: This is the first report of a CA being post-translationally regulated and describing phosphorylation events in the thylakoid lumen.
Conflict of interest statement
Figures


References
-
- Moroney JV, Ma Y, Frey WD, Fusilie KA, Pham TT, et al. (2011) The carbonic anhydrase isoforms of Chlamydomonas reinhardtii: intracellular location, expression, and physiological roles. Photosynth Res 109: 133–49. - PubMed
-
- Giordano M, Beardall J, Raven JA (2005) CO2 concentrating mechanisms in algae: mechanisms, environmental modulation and evolution. Annu Rev Plant Biol 56: 99–131. - PubMed
-
- Raven JA (1995) Phycological Reviews. Photosynthetic and non-photosynthetic roles of carbonic anhydrase in algae and cyanobacteria. Phycologia 34: 93–101.

