Targeted drug delivery for cancer therapy: the other side of antibodies
- PMID: 23140144
- PMCID: PMC3508879
- DOI: 10.1186/1756-8722-5-70
Targeted drug delivery for cancer therapy: the other side of antibodies
Abstract
Therapeutic monoclonal antibody (TMA) based therapies for cancer have advanced significantly over the past two decades both in their molecular sophistication and clinical efficacy. Initial development efforts focused mainly on humanizing the antibody protein to overcome problems of immunogenicity and on expanding of the target antigen repertoire. In parallel to naked TMAs, antibody-drug conjugates (ADCs) have been developed for targeted delivery of potent anti-cancer drugs with the aim of bypassing the morbidity common to conventional chemotherapy. This paper first presents a review of TMAs and ADCs approved for clinical use by the FDA and those in development, focusing on hematological malignancies. Despite advances in these areas, both TMAs and ADCs still carry limitations and we highlight the more important ones including cancer cell specificity, conjugation chemistry, tumor penetration, product heterogeneity and manufacturing issues. In view of the recognized importance of targeted drug delivery strategies for cancer therapy, we discuss the advantages of alternative drug carriers and where these should be applied, focusing on peptide-drug conjugates (PDCs), particularly those discovered through combinatorial peptide libraries. By defining the advantages and disadvantages of naked TMAs, ADCs and PDCs it should be possible to develop a more rational approach to the application of targeted drug delivery strategies in different situations and ultimately, to a broader basket of more effective therapies for cancer patients.
Figures
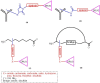
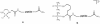
References
-
- Yamada T. Therapeutic monoclonal antibodies. Keio J Med. 2011;60(2):37–46. doi: 10.2302/kjm.60.37. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21720199. - DOI - PubMed
-
- Gellerman G, Firer MA. In: Targeted Drug Delivery in Cancer Therapeutics. Firer MA, editor. Transworld Research Network, Kerala; 2010. Targeted dendrimers in cancer drug delivery systems; pp. 185–209.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

