Novel antiviral activity of baicalein against dengue virus
- PMID: 23140177
- PMCID: PMC3528482
- DOI: 10.1186/1472-6882-12-214
Novel antiviral activity of baicalein against dengue virus
Abstract
Background: Dengue is a serious arboviral disease currently with no effective antiviral therapy or approved vaccine available. Therefore, finding the effective compound against dengue virus (DENV) replication is very important. Among the natural compounds, bioflavonoids derived mainly from plants are of interest because of their biological and medicinal benefits.
Methods: In the present study, antiviral activity of a bioflavonoid, baicalein, was evaluated against different stages of dengue virus type 2 (DENV-2) replication in Vero cells using focus forming unit reduction assay and quantitative RT-PCR.
Results: Baicalein inhibited DENV-2 replication in Vero cells with IC50= 6.46 μg/mL and SI= 17.8 when added after adsorption to the cells. The IC50 against DENV-2 was 5.39 μg/mL and SI= 21.3 when cells were treated 5 hours before virus infection and continuously up to 4 days post infection. Baicalein exhibited direct virucidal effect against DENV-2 with IC 50= 1.55 μg/mL and showed anti-adsorption effect with IC50 = 7.14 μg/mL.
Conclusions: Findings presented here suggest that baicalein exerts potent antiviral activity against DENV. Baicalein possesses direct virucidal activity against DENV besides its effects against dengue virus adsorption and intracellular replication of DENV-2. Baicalein, hence, should be considered for in vivo evaluation in the development of an effective antiviral compound against DENV.
Figures

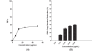
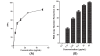
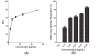

References
-
- AbuBakar S, Shafee N. Outlook of dengue in Malaysia: a century later. Malays J Pathol. 2002;24:23–27. - PubMed
-
- WHO. Dengue and Dengue Haemorrhagic Fever. Geneva: World Health Organisation; 2002. Fact Sheet No. 117.
-
- Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Endy TP, Raengsakulrach B, Rothman AL, Ennis FA, Nisalak A. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181:2–9. doi: 10.1086/315215. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

