Potential of primary kidney cells for somatic cell nuclear transfer mediated transgenesis in pig
- PMID: 23140586
- PMCID: PMC3537537
- DOI: 10.1186/1472-6750-12-84
Potential of primary kidney cells for somatic cell nuclear transfer mediated transgenesis in pig
Abstract
Background: Somatic cell nuclear transfer (SCNT) is currently the most efficient and precise method to generate genetically tailored pig models for biomedical research. However, the efficiency of this approach is crucially dependent on the source of nuclear donor cells. In this study, we evaluate the potential of primary porcine kidney cells (PKCs) as cell source for SCNT, including their proliferation capacity, transfection efficiency, and capacity to support full term development of SCNT embryos after additive gene transfer or homologous recombination.
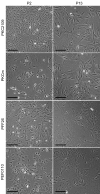
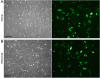
Results: PKCs could be maintained in culture with stable karyotype for up to 71 passages, whereas porcine fetal fibroblasts (PFFs) and porcine ear fibroblasts (PEFs) could be hardly passaged more than 20 times. Compared with PFFs and PEFs, PKCs exhibited a higher proliferation rate and resulted in a 2-fold higher blastocyst rate after SCNT and in vitro cultivation. Among the four transfection methods tested with a GFP expression plasmid, best results were obtained with the NucleofectorTM technology, resulting in transfection efficiencies of 70% to 89% with high fluorescence intensity, low cytotoxicity, good cell proliferation, and almost no morphological signs of cell stress. Usage of genetically modified PKCs in SCNT resulted in approximately 150 piglets carrying at least one of 18 different transgenes. Several of those pigs originated from PKCs that underwent homologous recombination and antibiotic selection before SCNT.
Conclusion: The high proliferation capacity of PKCs facilitates the introduction of precise and complex genetic modifications in vitro. PKCs are thus a valuable cell source for the generation of porcine biomedical models by SCNT.
Figures


References
-
- Klymiuk N, Aigner B, Brem G, Wolf E. Genetic modification of pigs as organ donors for xenotransplantation. Mol Reprod Dev. 2010;77:209–221. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

