Beyond gel electrophoresis: microfluidic separations, fluorescence burst analysis, and DNA stretching
- PMID: 23140825
- PMCID: PMC3595390
- DOI: 10.1021/cr3002142
Beyond gel electrophoresis: microfluidic separations, fluorescence burst analysis, and DNA stretching
Figures




































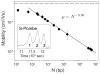
















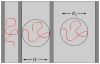
















References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

