The spinocerebellar ataxia-associated gene Tau tubulin kinase 2 controls the initiation of ciliogenesis
- PMID: 23141541
- PMCID: PMC3496184
- DOI: 10.1016/j.cell.2012.10.010
The spinocerebellar ataxia-associated gene Tau tubulin kinase 2 controls the initiation of ciliogenesis
Abstract
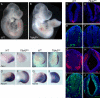
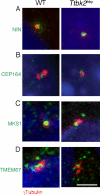
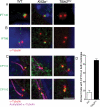
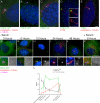
The primary cilium has critical roles in human development and disease, but the mechanisms that regulate ciliogenesis are not understood. Here, we show that Tau tubulin kinase 2 (TTBK2) is a dedicated regulator of the initiation of ciliogenesis in vivo. We identified a null allele of mouse Ttbk2 based on loss of Sonic hedgehog activity, a signaling pathway that requires the primary cilium. Despite a normal basal body template, Ttbk2 mutants lack cilia. TTBK2 acts at the distal end of the basal body, where it promotes the removal of CP110, which caps the mother centriole, and promotes recruitment of IFT proteins, which build the ciliary axoneme. Dominant truncating mutations in human TTBK2 cause spinocerebellar ataxia type 11 (SCA11); these mutant proteins do not promote ciliogenesis and inhibit ciliogenesis in wild-type cells. We propose that cell-cycle regulators target TTBK2 to the basal body, where it modifies specific targets to initiate ciliogenesis.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

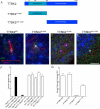
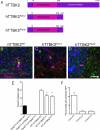
Comment in
-
TTBK2 kinase: linking primary cilia and cerebellar ataxias.Cell. 2012 Nov 9;151(4):697-699. doi: 10.1016/j.cell.2012.10.027. Cell. 2012. PMID: 23141531
References
-
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. - PubMed
-
- Aughsteen AA. The ultrastructure of primary cilia in the endocrine and excretory duct cells of the pancreas of mice and rats. Eur J Morphol. 2001;39:277–283. - PubMed
-
- Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. - PubMed
-
- Bauer P, Stevanin G, Beetz C, Synofzik M, Schmitz-Hubsch T, Wullner U, Berthier E, Ollagnon-Roman E, Riess O, Forlani S, et al. Spinocerebellar ataxia type 11 (SCA11) is an uncommon cause of dominant ataxia among French and German kindreds. J Neurol Neurosurg Ps. 2010;81:1229–1232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

