Recent advances in protein prenyltransferases: substrate identification, regulation, and disease interventions
- PMID: 23141597
- PMCID: PMC3518607
- DOI: 10.1016/j.cbpa.2012.10.015
Recent advances in protein prenyltransferases: substrate identification, regulation, and disease interventions
Abstract
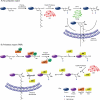
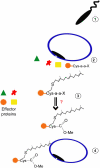
Protein post-translational modifications increase the functional diversity of the proteome by covalently adding chemical moieties onto proteins thereby changing their activation state, cellular localization, interacting partners, and life cycle. Lipidation is one such modification that enables membrane association of naturally cytosolic proteins. Protein prenyltransferases irreversibly install isoprenoid units of varying length via a thioether linkage onto proteins that exert their cellular activity at membranes. Substrates of prenyltransferases are involved in countless signaling pathways and processes within the cell. Identification of new prenylation substrates, prenylation pathway regulators, and dynamic trafficking of prenylated proteins are all avenues of intense, ongoing research that are challenging, exciting, and have the potential to significantly advance the field in the near future.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

