Nicotinic acetylcholine receptors containing the α4 subunit modulate alcohol reward
- PMID: 23141806
- PMCID: PMC4501776
- DOI: 10.1016/j.biopsych.2012.09.019
Nicotinic acetylcholine receptors containing the α4 subunit modulate alcohol reward
Abstract
Background: Nicotine and alcohol are the two most co-abused drugs in the world, suggesting a common mechanism of action might underlie their rewarding properties. Although nicotine elicits reward by activating ventral tegmental area dopaminergic (DAergic) neurons via high-affinity neuronal nicotinic acetylcholine receptors (nAChRs), the mechanism by which alcohol activates these neurons is unclear.
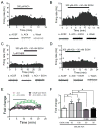
Methods: Because most high-affinity nAChRs expressed in ventral tegmental area DAergic neurons contain the α4 subunit, we measured ethanol-induced activation of DAergic neurons in midbrain slices from two complementary mouse models, an α4 knock-out (KO) mouse line and a knock-in line (Leu9'Ala) expressing α4 subunit-containing nAChRs hypersensitive to agonist compared with wild-type (WT). Activation of DAergic neurons by ethanol was analyzed with both biophysical and immunohistochemical approaches in midbrain slices. The ability of alcohol to condition a place preference in each mouse model was also measured.
Results: At intoxicating concentrations, ethanol activation of DAergic neurons was significantly reduced in α4 KO mice compared with WT. Conversely, in Leu9'Ala mice, DAergic neurons were activated by low ethanol concentrations that did not increase activity of WT neurons. In addition, alcohol potentiated the response to ACh in DAergic neurons, an effect reduced in α4 KO mice. Rewarding alcohol doses failed to condition a place preference in α4 KO mice, paralleling alcohol effects on DAergic neuron activity, whereas a sub-rewarding alcohol dose was sufficient to condition a place preference in Leu9'Ala mice.
Conclusions: Together, these data indicate that nAChRs containing the α4 subunit modulate alcohol reward.
Copyright © 2013 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Financial Disclosures. The authors report no biomedical financial interests or potential conflicts of interest.
Figures




Similar articles
-
Nicotinic acetylcholine receptors containing the α6 subunit contribute to ethanol activation of ventral tegmental area dopaminergic neurons.Biochem Pharmacol. 2013 Oct 15;86(8):1194-200. doi: 10.1016/j.bcp.2013.06.015. Epub 2013 Jun 26. Biochem Pharmacol. 2013. PMID: 23811312 Free PMC article.
-
Nicotine-mediated activation of dopaminergic neurons in distinct regions of the ventral tegmental area.Neuropsychopharmacology. 2011 Apr;36(5):1021-32. doi: 10.1038/npp.2010.240. Epub 2011 Feb 2. Neuropsychopharmacology. 2011. PMID: 21289604 Free PMC article.
-
Activation of alpha4* nAChRs is necessary and sufficient for varenicline-induced reduction of alcohol consumption.J Neurosci. 2010 Jul 28;30(30):10169-76. doi: 10.1523/JNEUROSCI.2601-10.2010. J Neurosci. 2010. PMID: 20668200 Free PMC article.
-
Neurochemical and behavioral studies on ethanol and nicotine interactions.Neurosci Biobehav Rev. 2004 Jan;27(8):713-20. doi: 10.1016/j.neubiorev.2003.11.010. Neurosci Biobehav Rev. 2004. PMID: 15019421 Review.
-
Mysterious alpha6-containing nAChRs: function, pharmacology, and pathophysiology.Acta Pharmacol Sin. 2009 Jun;30(6):740-51. doi: 10.1038/aps.2009.63. Acta Pharmacol Sin. 2009. PMID: 19498417 Free PMC article. Review.
Cited by
-
Ethanol-Induced Motor Impairment Mediated by Inhibition of α7 Nicotinic Receptors.J Neurosci. 2016 Jul 20;36(29):7768-78. doi: 10.1523/JNEUROSCI.0154-16.2016. J Neurosci. 2016. PMID: 27445152 Free PMC article.
-
Gene editing vectors for studying nicotinic acetylcholine receptors in cholinergic transmission.Eur J Neurosci. 2019 Aug;50(3):2224-2238. doi: 10.1111/ejn.13957. Epub 2018 Jul 25. Eur J Neurosci. 2019. PMID: 29779223 Free PMC article.
-
Nicotine Increases Alcohol Intake in Adolescent Male Rats.Front Behav Neurosci. 2017 Feb 22;11:25. doi: 10.3389/fnbeh.2017.00025. eCollection 2017. Front Behav Neurosci. 2017. PMID: 28275339 Free PMC article.
-
DNA damage and oxidative stress induced by seizures are decreased by anticonvulsant and neuroprotective effects of lobeline, a candidate to treat alcoholism.Metab Brain Dis. 2018 Feb;33(1):53-61. doi: 10.1007/s11011-017-0130-1. Epub 2017 Oct 14. Metab Brain Dis. 2018. PMID: 29032429
-
"Unraveling the role of CHRNA6, the neuronal α6 nicotinic acetylcholine receptor subunit".Receptors (Basel). 2025 Mar;4(1):1. doi: 10.3390/receptors4010001. Epub 2025 Jan 14. Receptors (Basel). 2025. PMID: 40331132 Free PMC article.
References
-
- Batel P, Pessione F, Maitre C, Rueff B. Relationship between alcohol and tobacco dependencies among alcoholics who smoke. Addiction. 1995;90:977–980. - PubMed
-
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, Melton LJ., 3rd Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. JAMA. 1996;275:1097–1103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

