Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study
- PMID: 23141813
- PMCID: PMC4347805
- DOI: 10.1016/S0140-6736(12)61770-X
Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study
Abstract
Background: LDL cholesterol (LDL-C) is a well established risk factor for cardiovascular disease. Proprotein convertase subtilisin/kexin type 9 (PCSK9) binds LDL receptors, targeting them for degradation. We therefore assessed the efficacy, safety, and tolerability of AMG 145, a human monoclonal IgG2 antibody against PCSK9, in stable patients with hypercholesterolemia on a statin.
Methods: In a phase 2, dose-ranging study done in 78 centres in the USA, Canada, Denmark, Hungary, and Czech Republic, patients (aged 18-80 years) with LDL-C greater than 2·2 mmol/L on a stable dose of statin (with or without ezetimibe), were randomly assigned equally, through an interactive voice response system, to subcutaneous injections of AMG 145 70 mg, 105 mg, or 140 mg, or matching placebo every 2 weeks; or subcutaneous injections of AMG 145 280 mg, 350 mg, or 420 mg, or matching placebo every 4 weeks. Everyone was masked to treatment assignment within the every 2 weeks and every 4 weeks schedules. The primary endpoint was the percentage change in LDL-C concentration from baseline after 12 weeks. Analysis was by modified intention to treat. This study is registered with ClinicalTrials.gov, number NCT01380730.
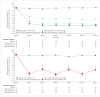
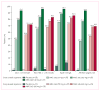
Findings: 631 patients with hypercholesterolaemia were randomly assigned to AMG 145 70 mg (n=79), 105 mg (n=79), or 140 mg (n=78), or matching placebo (n=78) every 2 weeks; or AMG 145 280 mg (n=79), 350 mg (n=79), and 420 mg (n=80), and matching placebo (n=79) every 4 weeks. At the end of the dosing interval at week 12, the mean LDL-C concentrations were reduced generally dose dependently by AMG 145 every 2 weeks (ranging from 41·8% to 66·1%; p<0·0001 for each dose vs placebo) and AMG 145 every 4 weeks (ranging from 41·8% to 50·3%; p<0·0001). No treatment-related serious adverse events occurred. The frequencies of treatment-related adverse events were similar in the AMG 145 and placebo groups (39 [8%] of 474 vs 11 [7%] of 155); none of these events were severe or life-threatening.
Interpretation: The results suggest that PCSK9 inhibition could be a new model in lipid management. Inhibition of PCSK9 warrants assessment in phase 3 clinical trials.
Funding: Amgen.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures

Comment in
-
Into the future: diversifying lipid management.Lancet. 2012 Dec 8;380(9858):1971-4. doi: 10.1016/S0140-6736(12)61888-1. Epub 2012 Nov 6. Lancet. 2012. PMID: 23141810 No abstract available.
-
Lipids. Antibodies against PCSK9--a new era of therapy.Nat Rev Cardiol. 2013 Jan;10(1):1. doi: 10.1038/nrcardio.2012.166. Epub 2012 Nov 20. Nat Rev Cardiol. 2013. PMID: 23165073 No abstract available.
-
Dyslipidaemia: PCSK9 antibodies: a dividend of the genomics revolution.Nat Rev Cardiol. 2013 Nov;10(11):618-9. doi: 10.1038/nrcardio.2013.139. Epub 2013 Sep 10. Nat Rev Cardiol. 2013. PMID: 24019032 No abstract available.
References
-
- O'Keefe JH, Jr, Cordain L, Harris WH, Moe RM, Vogel R. Optimal low-density lipoprotein is 50 to 70 mg/dl: lower is better and physiologically normal. J Am Coll Cardiol. 2004;43:2142–46. - PubMed
-
- Waters DD, Brotons C, Chiang CW, et al. Lipid treatment assessment project 2: a multinational survey to evaluate the proportion of patients achieving low-density lipoprotein cholesterol goals. Circulation. 2009;120:28–34. - PubMed
-
- Murphy SA, Cannon CP, Wiviott SD, McCabe CH, Braunwald E. Reduction in recurrent cardiovascular events with intensive lipid-lowering statin therapy compared with moderate lipid-lowering statin therapy after acute coronary syndromes from the PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction 22) trial. J Am Coll Cardiol. 2009;54:2358–62. - PubMed
-
- Artenstein AW, Opal SM. Proprotein convertases in health and disease. N Engl J Med. 2011;365:2507–18. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

