Impact of anti-orthopoxvirus neutralizing antibodies induced by a heterologous prime-boost HIV-1 vaccine on insert-specific immune responses
- PMID: 23142302
- PMCID: PMC3566524
- DOI: 10.1016/j.vaccine.2012.10.093
Impact of anti-orthopoxvirus neutralizing antibodies induced by a heterologous prime-boost HIV-1 vaccine on insert-specific immune responses
Abstract
Background: The impact of anti-vector immunity on the elicitation of insert-specific immune responses is important to understand in vaccine development. HVTN 055 was a 150 person phase I randomized, controlled HIV vaccine trial of recombinant modified vaccinia Ankara (rMVA) and fowlpox (rFPV) with matched HIV-1 inserts which demonstrated increased CD8+ T-cell immune responses in the heterologous vaccine group. The controls used in this study were the empty vectors (MVA and FPV).
Methods: Anti-MVA and anti-vaccinia neutralizing antibodies (NAbs) were measured and compared with cellular and humoral HIV-1-specific immune responses.
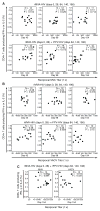
Results: Elicitation of anti-vector responses increased with increasing dose of MVA and up to 2 administrations. Further inoculations of MVA (up to 5) did not increase the magnitude of the anti-MVA response but did delay the anti-vector NAb titre decay. There was no evidence that the insert impaired the anti-vector response, nor that anti-vector immunity attenuated the insert-specific responses.
Conclusion: Two doses of MVA may be ideal for the elicitation of orthopoxvirus immune responses with further doses maintaining increased titres against the vector. We found no evidence that eliciting HIV insert- or MVA vector-specific immune responses interfered with elicitation of immune responses to the other.
Trial registration: ClinicalTrials.gov NCT00083603.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
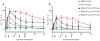
References
-
- Paris RM, Kim JH, Robb ML, Michael NL. Prime-boost immunization with poxvirus or adenovirus vectors as a strategy to develop a protective vaccine for HIV-1. Expert Rev Vaccines. 2010 Sep;9(9):1055–69. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

