Absence of venous valves in mice lacking Connexin37
- PMID: 23142761
- PMCID: PMC3533519
- DOI: 10.1016/j.ydbio.2012.10.032
Absence of venous valves in mice lacking Connexin37
Abstract
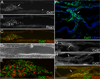
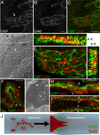
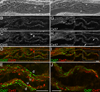
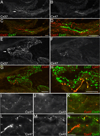
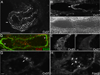
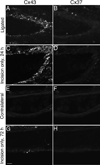
Venous valves play a crucial role in blood circulation, promoting the one-way movement of blood from superficial and deep veins towards the heart. By preventing retrograde flow, venous valves spare capillaries and venules from being subjected to damaging elevations in pressure, especially during skeletal muscle contraction. Pathologically, valvular incompetence or absence of valves are common features of venous disorders such as chronic venous insufficiency and varicose veins. The underlying causes of these conditions are not well understood, but congenital venous valve aplasia or agenesis may play a role in some cases. Despite progress in the study of cardiac and lymphatic valve morphogenesis, the molecular mechanisms controlling the development and maintenance of venous valves remain poorly understood. Here, we show that in valved veins of the mouse, three gap junction proteins (Connexins, Cxs), Cx37, Cx43, and Cx47, are expressed exclusively in the valves in a highly polarized fashion, with Cx43 on the upstream side of the valve leaflet and Cx37 on the downstream side. Surprisingly, Cx43 expression is strongly induced in the non-valve venous endothelium in superficial veins following wounding of the overlying skin. Moreover, we show that in Cx37-deficient mice, venous valves are entirely absent. Thus, Cx37, a protein involved in cell-cell communication, is one of only a few proteins identified so far as critical for the development or maintenance of venous valves. Because Cxs are necessary for the development of valves in lymphatic vessels as well, our results support the notion of common molecular pathways controlling valve development in veins and lymphatic vessels.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Segregated Foxc2, NFATc1 and Connexin expression at normal developing venous valves, and Connexin-specific differences in the valve phenotypes of Cx37, Cx43, and Cx47 knockout mice.Dev Biol. 2016 Apr 15;412(2):173-90. doi: 10.1016/j.ydbio.2016.02.033. Epub 2016 Mar 4. Dev Biol. 2016. PMID: 26953188 Free PMC article.
-
Connexin37 and Connexin43 deficiencies in mice disrupt lymphatic valve development and result in lymphatic disorders including lymphedema and chylothorax.Dev Biol. 2011 Jun 15;354(2):253-66. doi: 10.1016/j.ydbio.2011.04.004. Epub 2011 Apr 16. Dev Biol. 2011. PMID: 21515254 Free PMC article.
-
Mutations in EPHB4 cause human venous valve aplasia.JCI Insight. 2021 Sep 22;6(18):e140952. doi: 10.1172/jci.insight.140952. JCI Insight. 2021. PMID: 34403370 Free PMC article.
-
Flow control in our vessels: vascular valves make sure there is no way back.Cell Mol Life Sci. 2013 Mar;70(6):1055-66. doi: 10.1007/s00018-012-1110-6. Epub 2012 Aug 25. Cell Mol Life Sci. 2013. PMID: 22922986 Free PMC article. Review.
-
To what extent might deep venous thrombosis and chronic venous insufficiency share a common etiology?Int Angiol. 2009 Aug;28(4):254-68. Int Angiol. 2009. PMID: 19648868 Review.
Cited by
-
Intraluminal valves: development, function and disease.Dis Model Mech. 2017 Nov 1;10(11):1273-1287. doi: 10.1242/dmm.030825. Dis Model Mech. 2017. PMID: 29125824 Free PMC article. Review.
-
Human venous valve disease caused by mutations in FOXC2 and GJC2.J Exp Med. 2017 Aug 7;214(8):2437-2452. doi: 10.1084/jem.20160875. J Exp Med. 2017. PMID: 28724617 Free PMC article.
-
Mechanisms of Connexin-Related Lymphedema.Circ Res. 2018 Sep 28;123(8):964-985. doi: 10.1161/CIRCRESAHA.117.312576. Circ Res. 2018. PMID: 30355030 Free PMC article.
-
Connexin Expression Is Altered in Liver Development of Yotari (dab1 -/-) Mice.Int J Mol Sci. 2021 Oct 2;22(19):10712. doi: 10.3390/ijms221910712. Int J Mol Sci. 2021. PMID: 34639052 Free PMC article.
-
YAP and TAZ maintain PROX1 expression in the developing lymphatic and lymphovenous valves in response to VEGF-C signaling.Development. 2020 Dec 13;147(23):dev195453. doi: 10.1242/dev.195453. Development. 2020. PMID: 33060128 Free PMC article.
References
-
- Becker DL, Thrasivoulou C, Phillips AR. Connexins in wound healing; perspectives in diabetic patients. Biochim. Biophys. Acta. 2011;1818:2068–2075. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

