Recognition of the nonclassical MHC class I molecule H2-M3 by the receptor Ly49A regulates the licensing and activation of NK cells
- PMID: 23142773
- PMCID: PMC3913127
- DOI: 10.1038/ni.2468
Recognition of the nonclassical MHC class I molecule H2-M3 by the receptor Ly49A regulates the licensing and activation of NK cells
Erratum in
- Nat Immunol. 2013 Apr;14(4):413
Abstract
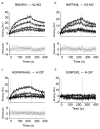
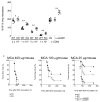
The development and function of natural killer (NK) cells is regulated by the interaction of inhibitory receptors of the Ly49 family with distinct peptide-laden major histocompatibility complex (MHC) class I molecules, although whether the Ly49 family is able bind to other MHC class I-like molecules is unclear. Here we found that the prototypic inhibitory receptor Ly49A bound the highly conserved nonclassical MHC class I molecule H2-M3 with an affinity similar to its affinity for H-2D(d). The specific recognition of H2-M3 by Ly49A regulated the 'licensing' of NK cells and mediated 'missing-self' recognition of H2-M3-deficient bone marrow. Host peptide-H2-M3 was required for optimal NK cell activity against experimental metastases and carcinogenesis. Thus, nonclassical MHC class I molecules can act as cognate ligands for Ly49 molecules. Our results provide insight into the various mechanisms that lead to NK cell tolerance.
Figures




Comment in
-
Nonclassical NK cell education.Nat Immunol. 2012 Dec;13(12):1135-7. doi: 10.1038/ni.2470. Nat Immunol. 2012. PMID: 23160207 No abstract available.
References
-
- Chan CJ, et al. Can NK cells be a therapeutic target in human cancer? Eur J Immunol. 2008;38:2964–2968. - PubMed
-
- Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol. 2007;7:329–339. - PubMed
-
- Andoniou CE, et al. Natural killer cells in viral infection: more than just killers. Immunol Rev. 2006;214:239–250. - PubMed
-
- Karlhofer FM, et al. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 1992;358:66–70. - PubMed
-
- Yu YY, et al. The role of Ly49A and 5E6(Ly49C) molecules in hybrid resistance mediated by murine natural killer cells against normal T cell blasts. Immunity. 1996;4:67–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

