A live, impaired-fidelity coronavirus vaccine protects in an aged, immunocompromised mouse model of lethal disease
- PMID: 23142821
- PMCID: PMC3518599
- DOI: 10.1038/nm.2972
A live, impaired-fidelity coronavirus vaccine protects in an aged, immunocompromised mouse model of lethal disease
Abstract
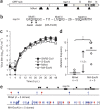
Live, attenuated RNA virus vaccines are efficacious but subject to reversion to virulence. Among RNA viruses, replication fidelity is recognized as a key determinant of virulence and escape from antiviral therapy; increased fidelity is attenuating for some viruses. Coronavirus (CoV) replication fidelity is approximately 20-fold greater than that of other RNA viruses and is mediated by a 3'→5' exonuclease (ExoN) activity that probably functions in RNA proofreading. In this study we demonstrate that engineered inactivation of severe acute respiratory syndrome (SARS)-CoV ExoN activity results in a stable mutator phenotype with profoundly decreased fidelity in vivo and attenuation of pathogenesis in young, aged and immunocompromised mice. The ExoN inactivation genotype and mutator phenotype are stable and do not revert to virulence, even after serial passage or long-term persistent infection in vivo. ExoN inactivation has potential for broad applications in the stable attenuation of CoVs and, perhaps, other RNA viruses.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Sessa R, Palagiano C, Scifoni MG, di Pietro M, Del Piano M. The major epidemic infections: a gift from the Old World to the New? Panminerva Med. 1999;41:78–84. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

