Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces
- PMID: 23142839
- PMCID: PMC3524530
- DOI: 10.1038/nmat3468
Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces
Abstract
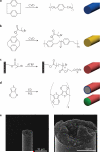
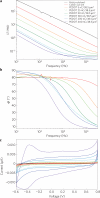
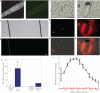
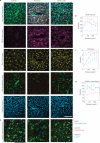
Implantable neural microelectrodes that can record extracellular biopotentials from small, targeted groups of neurons are critical for neuroscience research and emerging clinical applications including brain-controlled prosthetic devices. The crucial material-dependent problem is developing microelectrodes that record neural activity from the same neurons for years with high fidelity and reliability. Here, we report the development of an integrated composite electrode consisting of a carbon-fibre core, a poly(p-xylylene)-based thin-film coating that acts as a dielectric barrier and that is functionalized to control intrinsic biological processes, and a poly(thiophene)-based recording pad. The resulting implants are an order of magnitude smaller than traditional recording electrodes, and more mechanically compliant with brain tissue. They were found to elicit much reduced chronic reactive tissue responses and enabled single-neuron recording in acute and early chronic experiments in rats. This technology, taking advantage of new composites, makes possible highly selective and stealthy neural interface devices towards realizing long-lasting implants.
Figures


References
-
- Strumwasser F. Long-term recording’ from single neurons in brain of unrestrained mammals. Science. 1958;127:469–470. - PubMed
-
- Schmidt EM, Bak MJ, McIntosh JS. Long-term chronic recording from cortical neurons. Exp. Neurol. 1976;52:496–506. - PubMed
-
- Grill WM, Norman SE, Bellamkonda RV. Implanted neural interfaces: Biochallenges and engineered solutions. Annu. Rev. Biomed. Eng. 2009;11:1–24. - PubMed
-
- Biran R, Martin DC, Tresco PA. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp. Neurol. 2005;195:115–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

