Structure of the pre-60S ribosomal subunit with nuclear export factor Arx1 bound at the exit tunnel
- PMID: 23142978
- PMCID: PMC3678077
- DOI: 10.1038/nsmb.2438
Structure of the pre-60S ribosomal subunit with nuclear export factor Arx1 bound at the exit tunnel
Abstract
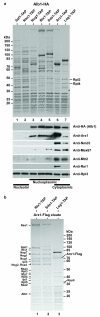
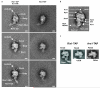
Preribosomal particles evolve in the nucleus through transient interaction with biogenesis factors before export to the cytoplasm. Here, we report the architecture of the late pre-60S particle, purified from Saccharomyces cerevisiae, through Arx1, a nuclear export factor with structural homology to methionine aminopeptidases, or its binding partner Alb1. Cryo-EM reconstruction of the Arx1 particle at 11.9-Å resolution reveals regions of extra density on the pre-60S particle attributed to associated biogenesis factors, confirming the immature state of the nascent subunit. One of these densities could be unambiguously assigned to Arx1. Immunoelectron microscopy and UV cross-linking localize Arx1 close to the ribosomal exit tunnel, in direct contact with ES27, a highly dynamic eukaryotic rRNA expansion segment. The binding of Arx1 at the exit tunnel may position this export factor to prevent premature recruitment of ribosome-associated factors active during translation.
Figures



References
-
- Kressler D, Hurt E, Bassler J. Driving ribosome assembly. Biochim Biophys Acta. 2010;1803:673–83. - PubMed
-
- Gorlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annual review of cell and developmental biology. 1999;15:607–60. - PubMed
-
- Zemp I, Kutay U. Nuclear export and cytoplasmic maturation of ribosomal subunits. FEBS Lett. 2007;581:2783–93. - PubMed
-
- Spahn CM, et al. Structure of the 80S ribosome from Saccharomyces cerevisiae--tRNA-ribosome and subunit-subunit interactions. Cell. 2001;107:373–86. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

