Cancer susceptibility and embryonic lethality in Mob1a/1b double-mutant mice
- PMID: 23143302
- PMCID: PMC3533542
- DOI: 10.1172/JCI63735
Cancer susceptibility and embryonic lethality in Mob1a/1b double-mutant mice
Abstract
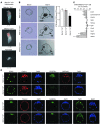
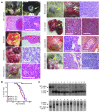
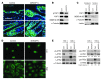
Mps one binder 1a (MOB1A) and MOB1B are key components of the Hippo signaling pathway and are mutated or inactivated in many human cancers. Here we show that intact Mob1a or Mob1b is essential for murine embryogenesis and that loss of the remaining WT Mob1 allele in Mob1a(Δ/Δ)1b(tr/+) or Mob1a(Δ/+)1b(tr/tr) mice results in tumor development. Because most of these cancers resembled trichilemmal carcinomas, we generated double-mutant mice bearing tamoxifen-inducible, keratinocyte-specific homozygous-null mutations of Mob1a and Mob1b (kDKO mice). kDKO mice showed hyperplastic keratinocyte progenitors and defective keratinocyte terminal differentiation and soon died of malnutrition. kDKO keratinocytes exhibited hyperproliferation, apoptotic resistance, impaired contact inhibition, enhanced progenitor self renewal, and increased centrosomes. Examination of Hippo pathway signaling in kDKO keratinocytes revealed that loss of Mob1a/b altered the activities of the downstream Hippo mediators LATS and YAP1. Similarly, YAP1 was activated in some human trichilemmal carcinomas, and some of these also exhibited MOB1A/1B inactivation. Our results clearly demonstrate that MOB1A and MOB1B have overlapping functions in skin homeostasis, and exert their roles as tumor suppressors by regulating downstream elements of the Hippo pathway.
Figures



References
-
- Fan H, Oro AE, Scott MP, Khavari PA. Induction of basal cell carcinoma features in transgenic human skin expressing Sonic Hedgehog. Nat Med. 1997;3(7):788–792. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

