Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses
- PMID: 23143305
- PMCID: PMC3533540
- DOI: 10.1172/JCI63287
Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses
Abstract
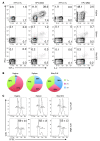
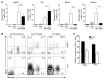
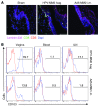
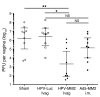
The induction of persistent intraepithelial CD8+ T cell responses may be key to the development of vaccines against mucosally transmitted pathogens, particularly for sexually transmitted diseases. Here we investigated CD8+ T cell responses in the female mouse cervicovaginal mucosa after intravaginal immunization with human papillomavirus vectors (HPV pseudoviruses) that transiently expressed a model antigen, respiratory syncytial virus (RSV) M/M2, in cervicovaginal keratinocytes. An HPV intravaginal prime/boost with different HPV serotypes induced 10-fold more cervicovaginal antigen-specific CD8+ T cells than priming alone. Antigen-specific T cell numbers decreased only 2-fold after 6 months. Most genital antigen-specific CD8+ T cells were intra- or subepithelial, expressed αE-integrin CD103, produced IFN-γ and TNF-α, and displayed in vivo cytotoxicity. Using a sphingosine-1-phosphate analog (FTY720), we found that the primed CD8+ T cells proliferated in the cervicovaginal mucosa upon HPV intravaginal boost. Intravaginal HPV prime/boost reduced cervicovaginal viral titers 1,000-fold after intravaginal challenge with vaccinia virus expressing the CD8 epitope M2. In contrast, intramuscular prime/boost with an adenovirus type 5 vector induced a higher level of systemic CD8+ T cells but failed to induce intraepithelial CD103+CD8+ T cells or protect against recombinant vaccinia vaginal challenge. Thus, HPV vectors are attractive gene-delivery platforms for inducing durable intraepithelial cervicovaginal CD8+ T cell responses by promoting local proliferation and retention of primed antigen-specific CD8+ T cells.
Figures







References
-
- Black CA, et al. Vaginal mucosa serves as an inductive site for tolerance. J Immunol. 2000;165(9):5077–5083. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

