Identification and prospective isolation of a mesothelial precursor lineage giving rise to smooth muscle cells and fibroblasts for mammalian internal organs, and their vasculature
- PMID: 23143399
- PMCID: PMC3685475
- DOI: 10.1038/ncb2610
Identification and prospective isolation of a mesothelial precursor lineage giving rise to smooth muscle cells and fibroblasts for mammalian internal organs, and their vasculature
Abstract
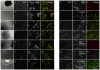
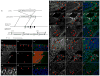
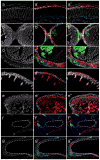
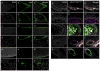
Fibroblasts and smooth muscle cells (FSMCs) are principal cell types of connective and adventitial tissues that participate in the development, physiology and pathology of internal organs, with incompletely defined cellular origins. Here, we identify and prospectively isolate from the mesothelium a mouse cell lineage that is committed to FSMCs. The mesothelium is an epithelial monolayer covering the vertebrate thoracic and abdominal cavities and internal organs. Time-lapse imaging and transplantation experiments reveal robust generation of FSMCs from the mesothelium. By targeting mesothelin (MSLN), a surface marker expressed on mesothelial cells, we identify and isolate precursors capable of clonally generating FSMCs. Using a genetic lineage tracing approach, we show that embryonic and adult mesothelium represents a common lineage to trunk FSMCs, and trunk vasculature, with minimal contributions from neural crest, or circulating cells. The isolation of FSMC precursors enables the examination of multiple aspects of smooth muscle and fibroblast biology as well as the prospective isolation of these precursors for potential regenerative medicine purposes.
Figures




Similar articles
-
The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature.Development. 2005 Dec;132(23):5317-28. doi: 10.1242/dev.02141. Development. 2005. PMID: 16284122
-
Lineage tracing using Wnt2b-2A-CreERT2 knock-in mice reveals the contributions of Wnt2b-expressing cells to novel subpopulations of mesothelial/epicardial cell lineages during mouse development.Genes Cells. 2024 Oct;29(10):854-875. doi: 10.1111/gtc.13147. Epub 2024 Aug 7. Genes Cells. 2024. PMID: 39109760
-
Wt1-expressing progenitors contribute to multiple tissues in the developing lung.Am J Physiol Lung Cell Mol Physiol. 2013 Aug 15;305(4):L322-32. doi: 10.1152/ajplung.00424.2012. Epub 2013 Jun 28. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23812634
-
Mesothelial-mesenchymal transitions in embryogenesis.Semin Cell Dev Biol. 2019 Aug;92:37-44. doi: 10.1016/j.semcdb.2018.09.006. Epub 2018 Sep 26. Semin Cell Dev Biol. 2019. PMID: 30243860 Review.
-
Cellular precursors of the coronary arteries.Tex Heart Inst J. 2002;29(4):243-9. Tex Heart Inst J. 2002. PMID: 12484607 Free PMC article. Review.
Cited by
-
Wt1, the mesothelium and the origins and heterogeneity of visceral fat progenitors.Adipocyte. 2015 Jan 29;4(3):217-21. doi: 10.4161/21623945.2014.985009. eCollection 2015 Jul-Sep. Adipocyte. 2015. PMID: 26257994 Free PMC article.
-
Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice.Elife. 2018 Sep 28;7:e39636. doi: 10.7554/eLife.39636. Elife. 2018. PMID: 30265241 Free PMC article.
-
Hand2 delineates mesothelium progenitors and is reactivated in mesothelioma.Nat Commun. 2022 Mar 30;13(1):1677. doi: 10.1038/s41467-022-29311-7. Nat Commun. 2022. PMID: 35354817 Free PMC article.
-
Derivation of lung mesenchymal lineages from the fetal mesothelium requires hedgehog signaling for mesothelial cell entry.Development. 2013 Nov;140(21):4398-406. doi: 10.1242/dev.098079. Development. 2013. PMID: 24130328 Free PMC article.
-
Autotaxin signaling governs phenotypic heterogeneity in visceral and parietal mesothelia.PLoS One. 2013 Jul 25;8(7):e69712. doi: 10.1371/journal.pone.0069712. Print 2013. PLoS One. 2013. PMID: 23936085 Free PMC article.
References
-
- Hay ED. Development of the vertebrate cornea. Int Rev Cytol. 1979;63:263–322. - PubMed
-
- Bochaton-Piallat ML, Gabbiani G. Smooth muscle cell: a key cell for plaque vulnerability regulation? Circ Res. 2006;98:448–9. - PubMed
-
- Kennedy LJ, Weissman IL. Dual origin of intimal cells in cardiac-allograft arteriosclerosis. N Engl J Med. 1971;285:884–887. - PubMed
-
- De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123:2229–38. - PubMed
-
- Desmoulière A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48:509–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

