Optical controlling reveals time-dependent roles for adult-born dentate granule cells
- PMID: 23143513
- PMCID: PMC3509272
- DOI: 10.1038/nn.3260
Optical controlling reveals time-dependent roles for adult-born dentate granule cells
Abstract
Accumulating evidence suggests that global depletion of adult hippocampal neurogenesis influences its function and that the timing of the depletion affects the deficits. However, the behavioral roles of adult-born neurons during their establishment of projections to CA3 pyramidal neurons remain largely unknown. We used a combination of retroviral and optogenetic approaches to birth date and reversibly control a group of adult-born neurons in adult mice. Adult-born neurons formed functional synapses on CA3 pyramidal neurons as early as 2 weeks after birth, and this projection to the CA3 area became stable by 4 weeks in age. Newborn neurons at this age were more plastic than neurons at other stages. Notably, we found that reversibly silencing this cohort of ~4-week-old cells after training, but not cells of other ages, substantially disrupted retrieval of hippocampal memory. Our results identify a restricted time window for adult-born neurons essential in hippocampal memory retrieval.
Figures
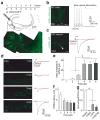


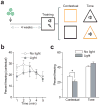
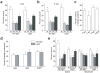
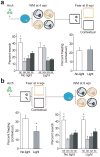
Comment in
-
New neurons retire early.Nat Neurosci. 2012 Dec;15(12):1611-2. doi: 10.1038/nn.3268. Nat Neurosci. 2012. PMID: 23187692 No abstract available.
Similar articles
-
New neurons retire early.Nat Neurosci. 2012 Dec;15(12):1611-2. doi: 10.1038/nn.3268. Nat Neurosci. 2012. PMID: 23187692 No abstract available.
-
Active Dentate Granule Cells Encode Experience to Promote the Addition of Adult-Born Hippocampal Neurons.J Neurosci. 2017 May 3;37(18):4661-4678. doi: 10.1523/JNEUROSCI.3417-16.2017. Epub 2017 Apr 3. J Neurosci. 2017. PMID: 28373391 Free PMC article.
-
Functional convergence of developmentally and adult-generated granule cells in dentate gyrus circuits supporting hippocampus-dependent memory.Hippocampus. 2011 Dec;21(12):1348-62. doi: 10.1002/hipo.20845. Epub 2010 Sep 7. Hippocampus. 2011. PMID: 20824726
-
Review: adult neurogenesis contributes to hippocampal plasticity.Cell Tissue Res. 2018 Sep;373(3):693-709. doi: 10.1007/s00441-017-2735-4. Epub 2017 Nov 29. Cell Tissue Res. 2018. PMID: 29185071 Review.
-
Critical periods regulating the circuit integration of adult-born hippocampal neurons.Cell Tissue Res. 2018 Jan;371(1):23-32. doi: 10.1007/s00441-017-2677-x. Epub 2017 Aug 22. Cell Tissue Res. 2018. PMID: 28828636 Review.
Cited by
-
New neurons retire early.Nat Neurosci. 2012 Dec;15(12):1611-2. doi: 10.1038/nn.3268. Nat Neurosci. 2012. PMID: 23187692 No abstract available.
-
Dynamic role of adult-born dentate granule cells in memory processing.Curr Opin Neurobiol. 2015 Dec;35:21-6. doi: 10.1016/j.conb.2015.06.002. Epub 2015 Jun 19. Curr Opin Neurobiol. 2015. PMID: 26100379 Free PMC article. Review.
-
Adult Neurogenesis, Context Encoding, and Pattern Separation: A Pathway for Treating Overgeneralization.Adv Neurobiol. 2024;38:163-193. doi: 10.1007/978-3-031-62983-9_10. Adv Neurobiol. 2024. PMID: 39008016 Review.
-
Neurogenesis Inhibition Prevents Enriched Environment to Prolong and Strengthen Social Recognition Memory, But Not to Increase BDNF Expression.Mol Neurobiol. 2017 Jul;54(5):3309-3316. doi: 10.1007/s12035-016-9922-2. Epub 2016 May 10. Mol Neurobiol. 2017. PMID: 27165290
-
Depletion of primary cilia from mature dentate granule cells impairs hippocampus-dependent contextual memory.Sci Rep. 2016 Sep 28;6:34370. doi: 10.1038/srep34370. Sci Rep. 2016. PMID: 27678193 Free PMC article.
References
-
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

