A chemical genetic approach reveals distinct EphB signaling mechanisms during brain development
- PMID: 23143520
- PMCID: PMC3509236
- DOI: 10.1038/nn.3249
A chemical genetic approach reveals distinct EphB signaling mechanisms during brain development
Abstract
EphB receptor tyrosine kinases control multiple steps in nervous system development. However, it remains unclear whether EphBs regulate these different developmental processes directly or indirectly. In addition, given that EphBs signal through multiple mechanisms, it has been challenging to define which signaling functions of EphBs regulate particular developmental events. To address these issues, we engineered triple knock-in mice in which the kinase activity of three neuronally expressed EphBs can be rapidly, reversibly and specifically blocked. We found that the tyrosine kinase activity of EphBs was required for axon guidance in vivo. In contrast, EphB-mediated synaptogenesis occurred normally when the kinase activity of EphBs was inhibited, suggesting that EphBs mediate synapse development by an EphB tyrosine kinase-independent mechanism. Taken together, our data indicate that EphBs control axon guidance and synaptogenesis by distinct mechanisms and provide a new mouse model for dissecting EphB function in development and disease.
Figures




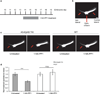
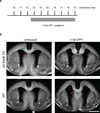


Comment in
-
To phosphorylate or not to phosphorylate: Selective alterations in tyrosine kinase-inhibited EphB mutant mice.Cell Adh Migr. 2014;8(1):1-4. doi: 10.4161/cam.27478. Epub 2013 Jan 1. Cell Adh Migr. 2014. PMID: 24589619 Free PMC article.
References
-
- Klein R. Eph/ephrin signaling in morphogenesis, neural development and plasticity. Curr Opin Cell Biol. 2004;16:580–589. - PubMed
-
- Lai KO, Ip NY. Synapse development and plasticity: roles of ephrin/Eph receptor signaling. Curr Opin Neurobiol. 2009;19:275–283. - PubMed
-
- Genander M, Frisen J. Ephrins and Eph receptors in stem cells and cancer. Curr Opin Cell Biol. 2010;22:611–616. - PubMed
-
- Merlos-Suarez A, Batlle E. Eph-ephrin signalling in adult tissues and cancer. Curr Opin Cell Biol. 2008;20:194–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

