Vertebrate kidney tubules elongate using a planar cell polarity-dependent, rosette-based mechanism of convergent extension
- PMID: 23143599
- PMCID: PMC4167614
- DOI: 10.1038/ng.2452
Vertebrate kidney tubules elongate using a planar cell polarity-dependent, rosette-based mechanism of convergent extension
Abstract
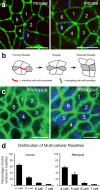
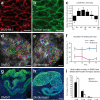
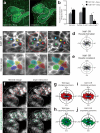
Cystic kidney diseases are a global public health burden, affecting over 12 million people. Although much is known about the genetics of kidney development and disease, the cellular mechanisms driving normal kidney tubule elongation remain unclear. Here, we used in vivo imaging to show for the first time that mediolaterally oriented cell intercalation is fundamental to vertebrate kidney morphogenesis. Unexpectedly, we found that kidney tubule elongation is driven in large part by a myosin-dependent, multicellular rosette-based mechanism, previously only described in Drosophila melanogaster. In contrast to findings in Drosophila, however, non-canonical Wnt and planar cell polarity (PCP) signaling is required to control rosette topology and orientation during vertebrate kidney tubule elongation. These data resolve long-standing questions concerning the role of PCP signaling in the developing kidney and, moreover, establish rosette-based intercalation as a deeply conserved cellular engine for epithelial morphogenesis.
Figures


References
-
- Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369:1287–301. - PubMed
-
- McNeill H. Planar cell polarity and the kidney. J Am Soc Nephrol. 2009;20:2104–11. - PubMed
-
- Costantini F. Renal branching morphogenesis: concepts, questions, and recent advances. Differentiation. 2006;74:402–21. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

