Long-term safety of pegloticase in chronic gout refractory to conventional treatment
- PMID: 23144450
- PMCID: PMC3756467
- DOI: 10.1136/annrheumdis-2012-201795
Long-term safety of pegloticase in chronic gout refractory to conventional treatment
Abstract
Objective: To evaluate the long-term safety (up to 3 years) of treatment with pegloticase in patients with refractory chronic gout.
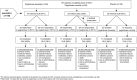
Methods: This open-label extension (OLE) study was conducted at 46 sites in the USA, Canada and Mexico. Patients completing either of two replicate randomised placebo-controlled 6-month trials received pegloticase 8 mg every 2 weeks (biweekly) or every 4 weeks (monthly). Safety was evaluated as the primary outcome, with special interest in gout flares and infusion-related reactions (IRs). Secondary outcomes included urate-lowering and clinical efficacy.
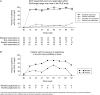
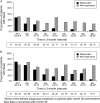
Results: Patients (n=149) received a mean±SD of 28±18 pegloticase infusions and were followed for a mean of 25±11 months. Gout flares and IRs were the most frequently reported adverse events; these were least common in patients with a sustained urate-lowering response to treatment and those receiving biweekly treatment. In 10 of the 11 patients with a serious IR, the event occurred when uric acid exceeded 6 mg/dl. Plasma and serum uric acid levels remained <6 mg/dl in most randomised controlled trial (RCT)-defined pegloticase responders throughout the OLE study and were accompanied by sustained and progressive improvements in tophus resolution and flare incidence.
Conclusions: The safety profile of long-term pegloticase treatment was consistent with that observed during 6 months of RCT treatment; no new safety signals were identified. Improvements in clinical status, in the form of flare and tophus reduction initiated during RCT pegloticase treatment in patients maintaining goal range urate-lowering responses were sustained or advanced during up to 2.5 years of additional treatment.
Keywords: Gout; Rheumatoid Arthritis; Treatment.
Figures

References
-
- Hamburger M, Baraf HS, Adamson TC, IIIet al. Recommendations for the diagnosis and management of gout and hyperuricemia. Postgrad Med 2011;123(6 Suppl 1):3–36 - PubMed
-
- Li-Yu J, Clayburne G, Sieck M, et al. Treatment of chronic gout. Can we determine when urate stores are depleted enough to prevent attacks of gout? J Rheumatol 2001;28:577–80 - PubMed
-
- Perez-Ruiz F, Lioté F. Lowering serum uric acid levels: what is the optimal target for improving clinical outcomes in gout? Arthritis Rheum 2007;57:1324–8 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

