Enhancing blockade of Plasmodium falciparum erythrocyte invasion: assessing combinations of antibodies against PfRH5 and other merozoite antigens
- PMID: 23144611
- PMCID: PMC3493472
- DOI: 10.1371/journal.ppat.1002991
Enhancing blockade of Plasmodium falciparum erythrocyte invasion: assessing combinations of antibodies against PfRH5 and other merozoite antigens
Abstract
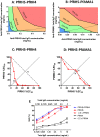
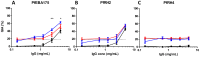
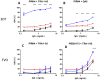
No vaccine has yet proven effective against the blood-stages of Plasmodium falciparum, which cause the symptoms and severe manifestations of malaria. We recently found that PfRH5, a P. falciparum-specific protein expressed in merozoites, is efficiently targeted by broadly-neutralizing, vaccine-induced antibodies. Here we show that antibodies against PfRH5 efficiently inhibit the in vitro growth of short-term-adapted parasite isolates from Cambodia, and that the EC(50) values of antigen-specific antibodies against PfRH5 are lower than those against PfAMA1. Since antibody responses elicited by multiple antigens are speculated to improve the efficacy of blood-stage vaccines, we conducted detailed assessments of parasite growth inhibition by antibodies against PfRH5 in combination with antibodies against seven other merozoite antigens. We found that antibodies against PfRH5 act synergistically with antibodies against certain other merozoite antigens, most notably with antibodies against other erythrocyte-binding antigens such as PfRH4, to inhibit the growth of a homologous P. falciparum clone. A combination of antibodies against PfRH4 and basigin, the erythrocyte receptor for PfRH5, also potently inhibited parasite growth. This methodology provides the first quantitative evidence that polyclonal vaccine-induced antibodies can act synergistically against P. falciparum antigens and should help to guide the rational development of future multi-antigen vaccines.
Conflict of interest statement
ARW, ADD, JJI, CC, GJW and SJD are named on patent applications relating to PfRH5 and/or other malaria vaccines. This does not alter our adherence to all PLoS Pathogens policies on sharing data and materials.
Figures



Similar articles
-
Bacterially expressed full-length recombinant Plasmodium falciparum RH5 protein binds erythrocytes and elicits potent strain-transcending parasite-neutralizing antibodies.Infect Immun. 2014 Jan;82(1):152-64. doi: 10.1128/IAI.00970-13. Epub 2013 Oct 14. Infect Immun. 2014. PMID: 24126527 Free PMC article.
-
Identification of a potent combination of key Plasmodium falciparum merozoite antigens that elicit strain-transcending parasite-neutralizing antibodies.Infect Immun. 2013 Feb;81(2):441-51. doi: 10.1128/IAI.01107-12. Epub 2012 Nov 26. Infect Immun. 2013. PMID: 23184525 Free PMC article.
-
Enhancing neutralization of Plasmodium falciparum using a novel monoclonal antibody against the rhoptry-associated membrane antigen.Sci Rep. 2022 Feb 23;12(1):3040. doi: 10.1038/s41598-022-06921-1. Sci Rep. 2022. PMID: 35197516 Free PMC article.
-
Antibodies and Plasmodium falciparum merozoites.Trends Parasitol. 2001 Apr;17(4):194-7. doi: 10.1016/s1471-4922(00)01946-2. Trends Parasitol. 2001. PMID: 11282510 Review.
-
The RH5-CyRPA-Ripr Complex as a Malaria Vaccine Target.Trends Parasitol. 2020 Jun;36(6):545-559. doi: 10.1016/j.pt.2020.04.003. Epub 2020 Apr 28. Trends Parasitol. 2020. PMID: 32359873 Free PMC article. Review.
Cited by
-
Plasmodium falciparum erythrocyte invasion: combining function with immune evasion.PLoS Pathog. 2014 Mar 20;10(3):e1003943. doi: 10.1371/journal.ppat.1003943. eCollection 2014 Mar. PLoS Pathog. 2014. PMID: 24651270 Free PMC article. Review.
-
Biosensor binding data and its applicability to the determination of active concentration.Biophys Rev. 2016 Dec;8(4):347-358. doi: 10.1007/s12551-016-0219-5. Epub 2016 Oct 17. Biophys Rev. 2016. PMID: 28510014 Free PMC article. Review.
-
Heterotypic interactions drive antibody synergy against a malaria vaccine candidate.Nat Commun. 2022 Feb 17;13(1):933. doi: 10.1038/s41467-022-28601-4. Nat Commun. 2022. PMID: 35177602 Free PMC article.
-
Naturally acquired antibodies specific for Plasmodium falciparum reticulocyte-binding protein homologue 5 inhibit parasite growth and predict protection from malaria.J Infect Dis. 2014 Mar 1;209(5):789-98. doi: 10.1093/infdis/jit553. Epub 2013 Oct 16. J Infect Dis. 2014. PMID: 24133188 Free PMC article.
-
Serological Profiling for Malaria Surveillance Using a Standard ELISA Protocol.Methods Mol Biol. 2019;2013:83-90. doi: 10.1007/978-1-4939-9550-9_6. Methods Mol Biol. 2019. PMID: 31267495 Free PMC article.
References
-
- World Health Organization (2011) World malaria report. World Health Organization, 2011.
-
- Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, et al. (2012) Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 379: 413–431. - PubMed
-
- Goodman AL, Draper SJ (2010) Blood-stage malaria vaccines - recent progress and future challenges. Ann Trop Med Parasitol 104: 189–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

