The polymorphic pseudokinase ROP5 controls virulence in Toxoplasma gondii by regulating the active kinase ROP18
- PMID: 23144612
- PMCID: PMC3493473
- DOI: 10.1371/journal.ppat.1002992
The polymorphic pseudokinase ROP5 controls virulence in Toxoplasma gondii by regulating the active kinase ROP18
Abstract
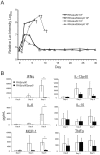
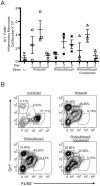
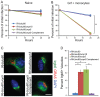
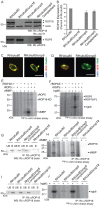
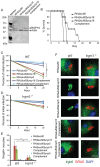
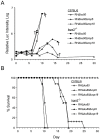
Secretory polymorphic serine/threonine kinases control pathogenesis of Toxoplasma gondii in the mouse. Genetic studies show that the pseudokinase ROP5 is essential for acute virulence, but do not reveal its mechanism of action. Here we demonstrate that ROP5 controls virulence by blocking IFN-γ mediated clearance in activated macrophages. ROP5 was required for the catalytic activity of the active S/T kinase ROP18, which phosphorylates host immunity related GTPases (IRGs) and protects the parasite from clearance. ROP5 directly regulated activity of ROP18 in vitro, and both proteins were necessary to avoid IRG recruitment and clearance in macrophages. Clearance of both the Δrop5 and Δrop18 mutants was reversed in macrophages lacking Irgm3, which is required for IRG function, and the virulence defect was fully restored in Irgm3(-/-) mice. Our findings establish that the pseudokinase ROP5 controls the activity of ROP18, thereby blocking IRG mediated clearance in macrophages. Additionally, ROP5 has other functions that are also Irgm3 and IFN-γ dependent, indicting it plays a general role in governing virulence factors that block immunity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
The rhoptry proteins ROP18 and ROP5 mediate Toxoplasma gondii evasion of the murine, but not the human, interferon-gamma response.PLoS Pathog. 2012;8(6):e1002784. doi: 10.1371/journal.ppat.1002784. Epub 2012 Jun 28. PLoS Pathog. 2012. PMID: 22761577 Free PMC article.
-
The Toxoplasma pseudokinase ROP5 forms complexes with ROP18 and ROP17 kinases that synergize to control acute virulence in mice.Cell Host Microbe. 2014 May 14;15(5):537-50. doi: 10.1016/j.chom.2014.04.002. Cell Host Microbe. 2014. PMID: 24832449 Free PMC article.
-
A Toxoplasma gondii pseudokinase inhibits host IRG resistance proteins.PLoS Biol. 2012;10(7):e1001358. doi: 10.1371/journal.pbio.1001358. Epub 2012 Jul 10. PLoS Biol. 2012. PMID: 22802726 Free PMC article.
-
The secreted kinase ROP18 defends Toxoplasma's border.Bioessays. 2011 Sep;33(9):693-700. doi: 10.1002/bies.201100054. Epub 2011 Jul 20. Bioessays. 2011. PMID: 21773979 Free PMC article. Review.
-
The IRG protein-based resistance mechanism in mice and its relation to virulence in Toxoplasma gondii.Curr Opin Microbiol. 2011 Aug;14(4):414-21. doi: 10.1016/j.mib.2011.07.002. Epub 2011 Jul 23. Curr Opin Microbiol. 2011. PMID: 21783405 Review.
Cited by
-
Secretion of Rhoptry and Dense Granule Effector Proteins by Nonreplicating Toxoplasma gondii Uracil Auxotrophs Controls the Development of Antitumor Immunity.PLoS Genet. 2016 Jul 22;12(7):e1006189. doi: 10.1371/journal.pgen.1006189. eCollection 2016 Jul. PLoS Genet. 2016. PMID: 27447180 Free PMC article.
-
IFNs in host defence and parasite immune evasion during Toxoplasma gondii infections.Front Immunol. 2024 Feb 7;15:1356216. doi: 10.3389/fimmu.2024.1356216. eCollection 2024. Front Immunol. 2024. PMID: 38384452 Free PMC article. Review.
-
Toxoplasma GRA15 and GRA24 are important activators of the host innate immune response in the absence of TLR11.PLoS Pathog. 2020 May 26;16(5):e1008586. doi: 10.1371/journal.ppat.1008586. eCollection 2020 May. PLoS Pathog. 2020. PMID: 32453782 Free PMC article.
-
Rhoptry Proteins ROP5 and ROP18 Are Major Murine Virulence Factors in Genetically Divergent South American Strains of Toxoplasma gondii.PLoS Genet. 2015 Aug 20;11(8):e1005434. doi: 10.1371/journal.pgen.1005434. eCollection 2015 Aug. PLoS Genet. 2015. PMID: 26291965 Free PMC article.
-
Influence of the Host and Parasite Strain on the Immune Response During Toxoplasma Infection.Front Cell Infect Microbiol. 2020 Oct 15;10:580425. doi: 10.3389/fcimb.2020.580425. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33178630 Free PMC article. Review.
References
-
- Dubey JP (2010) Toxoplasmosis of animals and humans. Boca Raton: CRC Press. 313 p.
-
- Howe DK, Sibley LD (1995) Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis 172: 1561–1566. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

