Mapping the phosphoproteome of influenza A and B viruses by mass spectrometry
- PMID: 23144613
- PMCID: PMC3493474
- DOI: 10.1371/journal.ppat.1002993
Mapping the phosphoproteome of influenza A and B viruses by mass spectrometry
Abstract
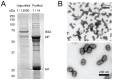
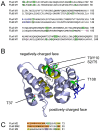
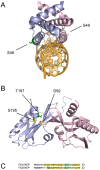
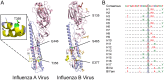
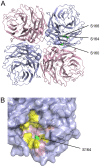
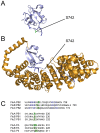
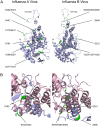
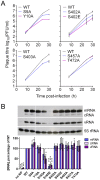
Protein phosphorylation is a common post-translational modification in eukaryotic cells and has a wide range of functional effects. Here, we used mass spectrometry to search for phosphorylated residues in all the proteins of influenza A and B viruses--to the best of our knowledge, the first time such a comprehensive approach has been applied to a virus. We identified 36 novel phosphorylation sites, as well as confirming 3 previously-identified sites. N-terminal processing and ubiquitination of viral proteins was also detected. Phosphorylation was detected in the polymerase proteins (PB2, PB1 and PA), glycoproteins (HA and NA), nucleoprotein (NP), matrix protein (M1), ion channel (M2), non-structural protein (NS1) and nuclear export protein (NEP). Many of the phosphorylation sites detected were conserved between influenza virus genera, indicating the fundamental importance of phosphorylation for all influenza viruses. Their structural context indicates roles for phosphorylation in regulating viral entry and exit (HA and NA); nuclear localisation (PB2, M1, NP, NS1 and, through NP and NEP, of the viral RNA genome); and protein multimerisation (NS1 dimers, M2 tetramers and NP oligomers). Using reverse genetics we show that for NP of influenza A viruses phosphorylation sites in the N-terminal NLS are important for viral growth, whereas mutating sites in the C-terminus has little or no effect. Mutating phosphorylation sites in the oligomerisation domains of NP inhibits viral growth and in some cases transcription and replication of the viral RNA genome. However, constitutive phosphorylation of these sites is not optimal. Taken together, the conservation, structural context and functional significance of phosphorylation sites implies a key role for phosphorylation in influenza biology. By identifying phosphorylation sites throughout the proteomes of influenza A and B viruses we provide a framework for further study of phosphorylation events in the viral life cycle and suggest a range of potential antiviral targets.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Lin YP, Gregory V, Bennett M, Hay A (2004) Recent changes among human influenza viruses. Virus Res 103: 47–52. - PubMed
-
- Buonagurio DA, Nakada S, Fitch WM, Palese P (1986) Epidemiology of influenza C virus in man: multiple evolutionary lineages and low rate of change. Virology 153: 12–21. - PubMed
-
- Cohen P (2000) The regulation of protein function by multisite phosphorylation–a 25 year update. Trends Biochem Sci 25: 596–601. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

