Vaccines: from empirical development to rational design
- PMID: 23144616
- PMCID: PMC3493475
- DOI: 10.1371/journal.ppat.1003001
Vaccines: from empirical development to rational design
Abstract
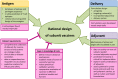
Infectious diseases are responsible for an overwhelming number of deaths worldwide and their clinical management is often hampered by the emergence of multi-drug-resistant strains. Therefore, prevention through vaccination currently represents the best course of action to combat them. However, immune escape and evasion by pathogens often render vaccine development difficult. Furthermore, most currently available vaccines were empirically designed. In this review, we discuss why rational design of vaccines is not only desirable but also necessary. We introduce recent developments towards specifically tailored antigens, adjuvants, and delivery systems, and discuss the methodological gaps and lack of knowledge still hampering true rational vaccine design. Finally, we address the potential and limitations of different strategies and technologies for advancing vaccine development.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Bagnoli F, Baudner B, Mishra RP, Bartolini E, Fiaschi L, et al. (2011) Designing the next generation of vaccines for global public health. OMICS 15: 545–566. - PubMed
-
- Poland GA, Kennedy RB, Ovsyannikova IG (2011) Vaccinomics and personalized vaccinology: is science leading us toward a new path of directed vaccine development and discovery? PLoS Pathog 7: e1002344 doi:10.1371/journal.ppat.1002344 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

