Early mechanisms of pathobiology are revealed by transcriptional temporal dynamics in hippocampal CA1 neurons of prion infected mice
- PMID: 23144617
- PMCID: PMC3493483
- DOI: 10.1371/journal.ppat.1003002
Early mechanisms of pathobiology are revealed by transcriptional temporal dynamics in hippocampal CA1 neurons of prion infected mice
Abstract
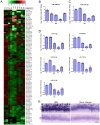
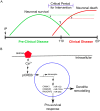
Prion diseases typically have long pre-clinical incubation periods during which time the infectious prion particle and infectivity steadily propagate in the brain. Abnormal neuritic sprouting and synaptic deficits are apparent during pre-clinical disease, however, gross neuronal loss is not detected until the onset of the clinical phase. The molecular events that accompany early neuronal damage and ultimately conclude with neuronal death remain obscure. In this study, we used laser capture microdissection to isolate hippocampal CA1 neurons and determined their pre-clinical transcriptional response during infection. We found that gene expression within these neurons is dynamic and characterized by distinct phases of activity. We found that a major cluster of genes is altered during pre-clinical disease after which expression either returns to basal levels, or alternatively undergoes a direct reversal during clinical disease. Strikingly, we show that this cluster contains a signature highly reminiscent of synaptic N-methyl-D-aspartic acid (NMDA) receptor signaling and the activation of neuroprotective pathways. Additionally, genes involved in neuronal projection and dendrite development were also altered throughout the disease, culminating in a general decline of gene expression for synaptic proteins. Similarly, deregulated miRNAs such as miR-132-3p, miR-124a-3p, miR-16-5p, miR-26a-5p, miR-29a-3p and miR-140-5p follow concomitant patterns of expression. This is the first in depth genomic study describing the pre-clinical response of hippocampal neurons to early prion replication. Our findings suggest that prion replication results in the persistent stimulation of a programmed response that is mediated, at least in part, by synaptic NMDA receptor activity that initially promotes cell survival and neurite remodelling. However, this response is terminated prior to the onset of clinical symptoms in the infected hippocampus, seemingly pointing to a critical juncture in the disease. Manipulation of these early neuroprotective pathways may redress the balance between degeneration and survival, providing a potential inroad for treatment.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
MicroRNA abundance is altered in synaptoneurosomes during prion disease.Mol Cell Neurosci. 2016 Mar;71:13-24. doi: 10.1016/j.mcn.2015.12.001. Epub 2015 Dec 4. Mol Cell Neurosci. 2016. PMID: 26658803
-
MicroRNA-16 targets mRNA involved in neurite extension and branching in hippocampal neurons during presymptomatic prion disease.Neurobiol Dis. 2018 Apr;112:1-13. doi: 10.1016/j.nbd.2017.12.011. Epub 2017 Dec 22. Neurobiol Dis. 2018. PMID: 29277556
-
Microdissection and transcriptional profiling: a window into the pathobiology of preclinical prion disease.Prion. 2014 Jan-Feb;8(1):67-74. doi: 10.4161/pri.27729. Prion. 2014. PMID: 24406429 Free PMC article.
-
Prion neurotoxicity.Brain Pathol. 2019 Mar;29(2):263-277. doi: 10.1111/bpa.12694. Epub 2019 Jan 17. Brain Pathol. 2019. PMID: 30588688 Free PMC article. Review.
-
[A process of programmed cell death as a mechanisms of neuronal death in prion diseases].Clin Exp Pathol. 1999;47(3-4):181-91. Clin Exp Pathol. 1999. PMID: 10472738 Review. French.
Cited by
-
miRNA Alterations Elicit Pathways Involved in Memory Decline and Synaptic Function in the Hippocampus of Aged Tg4-42 Mice.Front Neurosci. 2020 Sep 10;14:580524. doi: 10.3389/fnins.2020.580524. eCollection 2020. Front Neurosci. 2020. PMID: 33013313 Free PMC article.
-
MicroRNA-146a suppresses ROCK1 allowing hyperphosphorylation of tau in Alzheimer's disease.Sci Rep. 2016 May 25;6:26697. doi: 10.1038/srep26697. Sci Rep. 2016. PMID: 27221467 Free PMC article.
-
Mechanism and Therapeutic Prospect of miRNAs in Neurodegenerative Diseases.Behav Neurol. 2023 Nov 23;2023:8537296. doi: 10.1155/2023/8537296. eCollection 2023. Behav Neurol. 2023. PMID: 38058356 Free PMC article. Review.
-
Transcriptomic responses to prion disease in rats.BMC Genomics. 2015 Sep 5;16(1):682. doi: 10.1186/s12864-015-1884-7. BMC Genomics. 2015. PMID: 26341492 Free PMC article.
-
Prion Strain Differences in Accumulation of PrPSc on Neurons and Glia Are Associated with Similar Expression Profiles of Neuroinflammatory Genes: Comparison of Three Prion Strains.PLoS Pathog. 2016 Apr 5;12(4):e1005551. doi: 10.1371/journal.ppat.1005551. eCollection 2016 Apr. PLoS Pathog. 2016. PMID: 27046083 Free PMC article.
References
-
- Jeffrey M, Halliday WG, Bell J, Johnston AR, MacLeod NK, et al. (2000) Synapse loss associated with abnormal PrP precedes neuronal degeneration in the scrapie-infected murine hippocampus. Neuropathol Appl Neurobiol 26: 41–54. - PubMed
-
- Cunningham C, Deacon R, Wells H, Boche D, Waters S, et al. (2003) Synaptic changes characterize early behavioural signs in the ME7 model of murine prion disease. Eur J Neurosci 17: 2147–2155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

