A targeted glycan-related gene screen reveals heparan sulfate proteoglycan sulfation regulates WNT and BMP trans-synaptic signaling
- PMID: 23144627
- PMCID: PMC3493450
- DOI: 10.1371/journal.pgen.1003031
A targeted glycan-related gene screen reveals heparan sulfate proteoglycan sulfation regulates WNT and BMP trans-synaptic signaling
Abstract
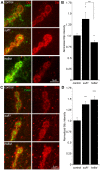
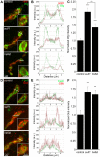
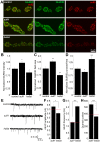
A Drosophila transgenic RNAi screen targeting the glycan genome, including all N/O/GAG-glycan biosynthesis/modification enzymes and glycan-binding lectins, was conducted to discover novel glycan functions in synaptogenesis. As proof-of-product, we characterized functionally paired heparan sulfate (HS) 6-O-sulfotransferase (hs6st) and sulfatase (sulf1), which bidirectionally control HS proteoglycan (HSPG) sulfation. RNAi knockdown of hs6st and sulf1 causes opposite effects on functional synapse development, with decreased (hs6st) and increased (sulf1) neurotransmission strength confirmed in null mutants. HSPG co-receptors for WNT and BMP intercellular signaling, Dally-like Protein and Syndecan, are differentially misregulated in the synaptomatrix of these mutants. Consistently, hs6st and sulf1 nulls differentially elevate both WNT (Wingless; Wg) and BMP (Glass Bottom Boat; Gbb) ligand abundance in the synaptomatrix. Anterograde Wg signaling via Wg receptor dFrizzled2 C-terminus nuclear import and retrograde Gbb signaling via synaptic MAD phosphorylation and nuclear import are differentially activated in hs6st and sulf1 mutants. Consequently, transcriptional control of presynaptic glutamate release machinery and postsynaptic glutamate receptors is bidirectionally altered in hs6st and sulf1 mutants, explaining the bidirectional change in synaptic functional strength. Genetic correction of the altered WNT/BMP signaling restores normal synaptic development in both mutant conditions, proving that altered trans-synaptic signaling causes functional differentiation defects.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Drosophila heparan sulfate 6-O-endosulfatase Sulf1 facilitates wingless (Wg) protein degradation.J Biol Chem. 2013 Feb 15;288(7):5081-9. doi: 10.1074/jbc.M112.447029. Epub 2013 Jan 7. J Biol Chem. 2013. PMID: 23300081 Free PMC article.
-
The role of Drosophila heparan sulfate 6-O-endosulfatase in sulfation compensation.J Biol Chem. 2013 Mar 1;288(9):6574-82. doi: 10.1074/jbc.M112.404830. Epub 2013 Jan 21. J Biol Chem. 2013. PMID: 23339195 Free PMC article.
-
Fragile X mental retardation protein regulates trans-synaptic signaling in Drosophila.Dis Model Mech. 2013 Nov;6(6):1400-13. doi: 10.1242/dmm.012229. Epub 2013 Sep 5. Dis Model Mech. 2013. PMID: 24046358 Free PMC article.
-
Extracellular heparan sulfate proteoglycans and glycan-binding lectins orchestrate trans-synaptic signaling.J Cell Sci. 2020 Aug 11;133(15):jcs244186. doi: 10.1242/jcs.244186. J Cell Sci. 2020. PMID: 32788209 Free PMC article. Review.
-
Genetic variability in proteoglycan biosynthetic genes reveals new facets of heparan sulfate diversity.Essays Biochem. 2024 Dec 4;68(4):555-578. doi: 10.1042/EBC20240106. Essays Biochem. 2024. PMID: 39630030 Free PMC article. Review.
Cited by
-
Retrograde BMP signaling at the synapse: a permissive signal for synapse maturation and activity-dependent plasticity.J Neurosci. 2013 Nov 6;33(45):17937-50. doi: 10.1523/JNEUROSCI.6075-11.2013. J Neurosci. 2013. PMID: 24198381 Free PMC article.
-
Secreted tissue inhibitor of matrix metalloproteinase restricts trans-synaptic signaling to coordinate synaptogenesis.J Cell Sci. 2017 Jul 15;130(14):2344-2358. doi: 10.1242/jcs.200808. Epub 2017 Jun 2. J Cell Sci. 2017. PMID: 28576972 Free PMC article.
-
Genetic background mutations drive neural circuit hyperconnectivity in a fragile X syndrome model.BMC Biol. 2020 Jul 30;18(1):94. doi: 10.1186/s12915-020-00817-0. BMC Biol. 2020. PMID: 32731855 Free PMC article.
-
Carrier of Wingless (Cow) Regulation of Drosophila Neuromuscular Junction Development.eNeuro. 2020 Mar 10;7(2):ENEURO.0285-19.2020. doi: 10.1523/ENEURO.0285-19.2020. Print 2020 Mar/Apr. eNeuro. 2020. PMID: 32024666 Free PMC article.
-
Two protein N-acetylgalactosaminyl transferases regulate synaptic plasticity by activity-dependent regulation of integrin signaling.J Neurosci. 2014 Sep 24;34(39):13047-65. doi: 10.1523/JNEUROSCI.1484-14.2014. J Neurosci. 2014. PMID: 25253852 Free PMC article.
References
-
- Iozzo RV (1998) Matrix proteoglycans: From molecular design to cellular function. Annual Review of Biochemistry 67: 609–652. - PubMed
-
- Kleene R, Schachner M (2004) Glycans and neural cell interactions. Nature Reviews Neuroscience 5: 195–208. - PubMed
-
- Dityatev A, Schachner M (2006) The extracellular matrix and synapses. Cell and Tissue Research 326: 647–654. - PubMed
-
- Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, et al.. (2009) Essentials of glycobiology. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

