Calcium regulation of keratinocyte differentiation
- PMID: 23144648
- PMCID: PMC3491811
- DOI: 10.1586/eem.12.34
Calcium regulation of keratinocyte differentiation
Abstract
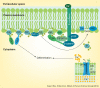
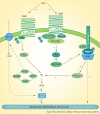
Calcium is the major regulator of keratinocyte differentiation in vivo and in vitro. A calcium gradient within the epidermis promotes the sequential differentiation of keratinocytes as they traverse the different layers of the epidermis to form the permeability barrier of the stratum corneum. Calcium promotes differentiation by both outside-in and inside-out signaling. A number of signaling pathways involved with differentiation are regulated by calcium, including the formation of desmosomes, adherens junctions and tight junctions, which maintain cell-cell adhesion and play an important intracellular signaling role through their activation of various kinases and phospholipases that produce second messengers that regulate intracellular free calcium and PKC activity, critical for the differentiation process. The calcium receptor plays a central role by initiating the intracellular signaling events that drive differentiation in response to extracellular calcium. This review will discuss these mechanisms.
Figures

References
-
- Green KJ, Simpson CL. Desmosomes: new perspectives on a classic. J. Invest. Dermatol. 2007;127(11):2499–2515. - PubMed
-
- Yuki T, Haratake A, Koishikawa H, Morita K, Miyachi Y, Inoue S. Tight junction proteins in keratinocytes: localization and contribution to barrier function. Exp. Dermatol. 2007;16(4):324–330. - PubMed
-
- Marchisio PC, Bondanza S, Cremona O, Cancedda R, De Luca M. Polarized expression of integrin receptors (α 6 β 4, α 2 β 1, α 3 β 1, and α v β 5) and their relationship with the cytoskeleton and basement membrane matrix in cultured human keratinocytes. J. Cell Biol. 1991;112(4):761–773. - PMC - PubMed
-
- Guo M, Kim LT, Akiyama SK, Gralnick HR, Yamada KM, Grinnell F. Altered processing of integrin receptors during keratinocyte activation. Exp. Cell Res. 1991;195(2):315–322. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
