Atrial tachyarrhythmia in Rgs5-null mice
- PMID: 23144791
- PMCID: PMC3489853
- DOI: 10.1371/journal.pone.0046856
Atrial tachyarrhythmia in Rgs5-null mice
Abstract
Aims: The aim of this study was to elucidate the effects of regulator of G-protein signaling 5 (Rgs5), a negative regulator of G protein-mediated signaling, on atrial repolarization and tachyarrhythmia (ATA) in mice.
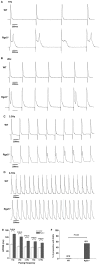
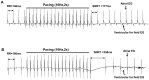
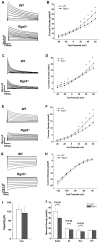
Methods and results: In present study, the incidence of ATA were increased in Rgs5(-/-) Langendorff-perfused mouse hearts during program electrical stimulation (PES) (46.7%, 7 of 15) and burst pacing (26.7%, 4 of 15) compared with wild-type (WT) mice (PES: 7.1%,1 of 14; burst:7.1%,1 of 14) (P<0.05). And the duration of ATA also shown longer in Rgs5(-/-) heart than that in WT, 2 out of 15 hearts exhibited sustained ATA (>30 s) but none of them observed in WT mice. Atrial prolonged repolarization was observed in Rgs5(-/-) hearts including widened P wave in surface ECG recording, increased action potential duration (APD) and atrial effective refractory periods (AERP), all of them showed significant difference with WT mice (P<0.05). At the cellular level, whole-cell patch clamp recorded markedly decreased densities of repolarizing K(+) currents including I(Kur) (at +60 mV: 14.0±2.2 pF/pA) and I(to) (at +60 mV: 16.7±1.3 pA/pF) in Rgs5(-/-) atrial cardiomyocytes, compared to those of WT mice (at +60 mV I(to): 20.4±2.0 pA/pF; I(kur): 17.9±2.0 pF/pA) (P<0.05).
Conclusion: These results suggest that Rgs5 is an important regulator of arrhythmogenesis in the mouse atrium and that the enhanced susceptibility to atrial tachyarrhythmias in Rgs5(-/-) mice may contribute to abnormalities of atrial repolarization.
Conflict of interest statement
Figures





References
-
- Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, et al. (1998) Impact of atrial fibrillation on the risk of death:the Framingham Heart Study. Circulation 98: 946–952. - PubMed
-
- Domenighetti AA, Boixel C, Cefai D, Abriel H, Pedrazzini T (2007) Chronic angiotensin II stimulation in the heart produces an acquired long QT syndrome associated with IK1 potassium current downregulation. J Mol Cell Cardiol 42: 63–70. - PubMed
-
- Chen YH, Xu SJ, Bendahhou S, Wang XL, Wang Y, et al. (2003) KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science 299: 251–254. - PubMed
-
- Xiao B, Zhang Y, Niu WQ, Gao PJ, Zhu DL (2009) Haplotype-based association of regulator of G-protein signaling 5 gene polymorphisms with essential hypertension and metabolic parameters. Clin Chem Lab Med 47: 1483–1488. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

