Use of anti-retroviral therapy in tuberculosis patients on second-line anti-TB regimens: a systematic review
- PMID: 23144818
- PMCID: PMC3489892
- DOI: 10.1371/journal.pone.0047370
Use of anti-retroviral therapy in tuberculosis patients on second-line anti-TB regimens: a systematic review
Abstract
Introduction: Use of antiretroviral therapy (ART) during treatment of drug susceptible tuberculosis (TB) improves survival. However, data from HIV infected individuals with drug resistant TB are lacking. Second line TB drugs when combined with ART may increase drug interactions and lead to higher rates of toxicity and greater noncompliance. This systematic review sought to determine the benefit of ART in the setting of second line drug therapy for drug resistant TB.
Methods: We included individual patient data from studies that evaluated treatment of drug-resistant tuberculosis in HIV-1 infected individuals published between January 1980 and December of 2009. We evaluated the effect of ART on treatment outcomes, time to smear and culture conversion, and adverse events.
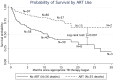
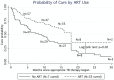
Results: Ten observational studies, including data from 217 subjects, were analyzed. Patients using ART during TB treatment had increased likelihood of cure (hazard ratio (HR) 3.4, 95% CI 1.6-7.4) and decreased likelihood of death (HR 0.4, 95% CI 0.3-0.6) during treatment for drug resistant TB. These associations remained significant in patients with a CD4 less than 200 cells/mm(3) and less than 50 cells/mm(3), and when correcting for drug resistance pattern.
Limitations: We identified only observational studies from which individual patient data could be drawn. Limitations in study design, and heterogeneity in a number of the outcomes of interest had the potential to introduce bias.
Discussion: While there are insufficient data to determine if ART use increases adverse drug interactions when used with second line TB drugs, ART use during treatment of drug resistant TB appears to improve cure rates and decrease risk of death. All individuals with HIV appear to benefit from ART use during treatment for TB.
Conflict of interest statement
Figures
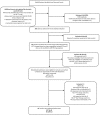
References
-
- WHO (2011) Global Tuberculosis Control: WHO report 2011. Geneva
-
- WHO (2010) Antiretroviral Therapy for HIV infection in adults and adolescents: recomendations for a public health approach 2010 revision. Geneva - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

