Inhibition of both protease and helicase activities of hepatitis C virus NS3 by an ethyl acetate extract of marine sponge Amphimedon sp
- PMID: 23144928
- PMCID: PMC3492463
- DOI: 10.1371/journal.pone.0048685
Inhibition of both protease and helicase activities of hepatitis C virus NS3 by an ethyl acetate extract of marine sponge Amphimedon sp
Abstract
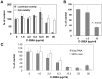
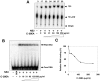
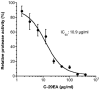
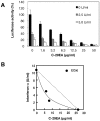
Combination therapy with ribavirin, interferon, and viral protease inhibitors could be expected to elicit a high level of sustained virologic response in patients infected with hepatitis C virus (HCV). However, several severe side effects of this combination therapy have been encountered in clinical trials. In order to develop more effective and safer anti-HCV compounds, we employed the replicon systems derived from several strains of HCV to screen 84 extracts from 54 organisms that were gathered from the sea surrounding Okinawa Prefecture, Japan. The ethyl acetate-soluble extract that was prepared from marine sponge Amphimedon sp. showed the highest inhibitory effect on viral replication, with EC₅₀ values of 1.5 and 24.9 µg/ml in sub-genomic replicon cell lines derived from genotypes 1b and 2a, respectively. But the extract had no effect on interferon-inducing signaling or cytotoxicity. Treatment with the extract inhibited virus production by 30% relative to the control in the JFH1-Huh7 cell culture system. The in vitro enzymological assays revealed that treatment with the extract suppressed both helicase and protease activities of NS3 with IC₅₀ values of 18.9 and 10.9 µg/ml, respectively. Treatment with the extract of Amphimedon sp. inhibited RNA-binding ability but not ATPase activity. These results suggest that the novel compound(s) included in Amphimedon sp. can target the protease and helicase activities of HCV NS3.
Conflict of interest statement
Figures




Similar articles
-
Hepatitis C Virus NS3 Protease and Helicase Inhibitors from Red Sea Sponge (Amphimedon) Species in Green Synthesized Silver Nanoparticles Assisted by in Silico Modeling and Metabolic Profiling.Int J Nanomedicine. 2020 May 12;15:3377-3389. doi: 10.2147/IJN.S233766. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32494136 Free PMC article.
-
[Preliminary screening of activity fraction of Alchornea trewioides suppresses expression of subgenomic hepatitis C virus RNA in vitro].Zhong Yao Cai. 2013 Jun;36(6):880-3. Zhong Yao Cai. 2013. PMID: 24380267 Chinese.
-
Inhibition of hepatitis C virus replication and viral helicase by ethyl acetate extract of the marine feather star Alloeocomatella polycladia.Mar Drugs. 2012 Apr;10(4):744-761. doi: 10.3390/md10040744. Epub 2012 Mar 28. Mar Drugs. 2012. PMID: 22690141 Free PMC article.
-
A review of HCV protease inhibitors.Curr Opin Investig Drugs. 2009 Aug;10(8):821-37. Curr Opin Investig Drugs. 2009. PMID: 19649927 Review.
-
TMC-435, an NS3/4A protease inhibitor for the treatment of HCV infection.Curr Opin Investig Drugs. 2009 Aug;10(8):871-81. Curr Opin Investig Drugs. 2009. PMID: 19649931 Review.
Cited by
-
Hepatitis C virus NS3 inhibitors: current and future perspectives.Biomed Res Int. 2013;2013:467869. doi: 10.1155/2013/467869. Epub 2013 Oct 27. Biomed Res Int. 2013. PMID: 24282816 Free PMC article. Review.
-
Inhibitory effects of caffeic acid phenethyl ester derivatives on replication of hepatitis C virus.PLoS One. 2013 Dec 17;8(12):e82299. doi: 10.1371/journal.pone.0082299. eCollection 2013. PLoS One. 2013. PMID: 24358168 Free PMC article.
-
Hepatitis C Virus NS3 Protease and Helicase Inhibitors from Red Sea Sponge (Amphimedon) Species in Green Synthesized Silver Nanoparticles Assisted by in Silico Modeling and Metabolic Profiling.Int J Nanomedicine. 2020 May 12;15:3377-3389. doi: 10.2147/IJN.S233766. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32494136 Free PMC article.
References
-
- Baldo V, Baldovin T, Trivello R, Floreani A (2008) Epidemiology of HCV infection. Curr Pharm Des 14: 1646–1654. - PubMed
-
- Seeff LB (2002) Natural history of chronic hepatitis C. Hepatology. 36: S35–46. - PubMed
-
- Kim DW, Gwack Y, Han JH, Choe J (1995) C-terminal domain of the hepatitis C virus NS3 protein contains an RNA helicase activity. Biochem Biophys Res Commun 215: 160–166. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

