Endogenous thrombospondin-1 regulates leukocyte recruitment and activation and accelerates death from systemic candidiasis
- PMID: 23144964
- PMCID: PMC3492437
- DOI: 10.1371/journal.pone.0048775
Endogenous thrombospondin-1 regulates leukocyte recruitment and activation and accelerates death from systemic candidiasis
Abstract
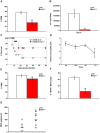
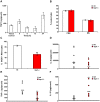
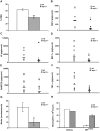
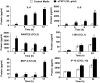
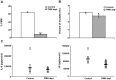
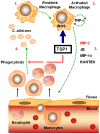
Disseminated Candida albicans infection results in high morbidity and mortality despite treatment with existing antifungal drugs. Recent studies suggest that modulating the host immune response can improve survival, but specific host targets for accomplishing this goal remain to be identified. The extracellular matrix protein thrombospondin-1 is released at sites of tissue injury and modulates several immune functions, but its role in C. albicans pathogenesis has not been investigated. Here, we show that mice lacking thrombospondin-1 have an advantage in surviving disseminated candidiasis and more efficiently clear the initial colonization from kidneys despite exhibiting fewer infiltrating leukocytes. By examining local and systemic cytokine responses to C. albicans and other standard inflammatory stimuli, we identify a crucial function of phagocytes in this enhanced resistance. Subcutaneous air pouch and systemic candidiasis models demonstrated that endogenous thrombospondin-1 enhances the early innate immune response against C. albicans and promotes activation of inflammatory macrophages (inducible nitric oxide synthase⁺, IL-6(high), TNF-α(high), IL-10(low)), release of the chemokines MIP-2, JE, MIP-1α, and RANTES, and CXCR2-driven polymorphonuclear leukocytes recruitment. However, thrombospondin-1 inhibited the phagocytic capacity of inflammatory leukocytes in vivo and in vitro, resulting in increased fungal burden in the kidney and increased mortality in wild type mice. Thus, thrombospondin-1 enhances the pathogenesis of disseminated candidiasis by creating an imbalance in the host immune response that ultimately leads to reduced phagocytic function, impaired fungal clearance, and increased mortality. Conversely, inhibitors of thrombospondin-1 may be useful drugs to improve patient recovery from disseminated candidiasis.
Conflict of interest statement
Figures

Similar articles
-
CD47 Promotes Protective Innate and Adaptive Immunity in a Mouse Model of Disseminated Candidiasis.PLoS One. 2015 May 26;10(5):e0128220. doi: 10.1371/journal.pone.0128220. eCollection 2015. PLoS One. 2015. PMID: 26010544 Free PMC article.
-
Increased susceptibility of TNF-alpha lymphotoxin-alpha double knockout mice to systemic candidiasis through impaired recruitment of neutrophils and phagocytosis of Candida albicans.J Immunol. 1999 Aug 1;163(3):1498-505. J Immunol. 1999. PMID: 10415052
-
Phagocytes from Mice Lacking the Sts Phosphatases Have an Enhanced Antifungal Response to Candida albicans.mBio. 2018 Jul 17;9(4):e00782-18. doi: 10.1128/mBio.00782-18. mBio. 2018. PMID: 30018105 Free PMC article.
-
Modulation of neutrophil function in host defense against disseminated Candida albicans infection in mice.FEMS Immunol Med Microbiol. 1999 Dec;26(3-4):299-307. doi: 10.1111/j.1574-695X.1999.tb01402.x. FEMS Immunol Med Microbiol. 1999. PMID: 10575142 Review.
-
Production and function of cytokines in natural and acquired immunity to Candida albicans infection.Microbiol Rev. 1995 Dec;59(4):646-72. doi: 10.1128/mr.59.4.646-672.1995. Microbiol Rev. 1995. PMID: 8531890 Free PMC article. Review.
Cited by
-
Molecular and Physiological Study of Candida albicans by Quantitative Proteome Analysis.Proteomes. 2018 Sep 18;6(3):34. doi: 10.3390/proteomes6030034. Proteomes. 2018. PMID: 30231513 Free PMC article. Review.
-
Candida albicans Elicits Pro-Inflammatory Differential Gene Expression in Intestinal Peyer's Patches.Mycopathologia. 2019 Aug;184(4):461-478. doi: 10.1007/s11046-019-00349-4. Epub 2019 Jun 22. Mycopathologia. 2019. PMID: 31230200
-
Differential intolerance to loss of function and missense mutations in genes that encode human matricellular proteins.J Cell Commun Signal. 2021 Mar;15(1):93-105. doi: 10.1007/s12079-020-00598-9. Epub 2021 Jan 7. J Cell Commun Signal. 2021. PMID: 33415696 Free PMC article.
-
Emerging functions of thrombospondin-1 in immunity.Semin Cell Dev Biol. 2024 Mar 1;155(Pt B):22-31. doi: 10.1016/j.semcdb.2023.05.008. Epub 2023 May 29. Semin Cell Dev Biol. 2024. PMID: 37258315 Free PMC article. Review.
-
Thrombospondins: old players, new games.Curr Opin Lipidol. 2013 Oct;24(5):401-9. doi: 10.1097/MOL.0b013e3283642912. Curr Opin Lipidol. 2013. PMID: 23892609 Free PMC article. Review.
References
-
- Raugi GJ, Olerud JE, Gown AM (1987) Thrombospondin in early human wound tissue. J Invest Dermatol 89: 551–554. - PubMed
-
- Gotis-Graham I, Hogg PJ, McNeil HP (1997) Significant correlation between thrombospondin 1 and serine proteinase expression in rheumatoid synovium. Arthritis Rheum 40: 1780–1787. - PubMed
-
- Mansfield PJ, Suchard SJ (1994) Thrombospondin promotes chemotaxis and haptotaxis of human peripheral blood monocytes. J Immunol 153: 4219–4229. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

