Protein tyrosine phosphatase receptor type z negatively regulates oligodendrocyte differentiation and myelination
- PMID: 23144976
- PMCID: PMC3492236
- DOI: 10.1371/journal.pone.0048797
Protein tyrosine phosphatase receptor type z negatively regulates oligodendrocyte differentiation and myelination
Abstract
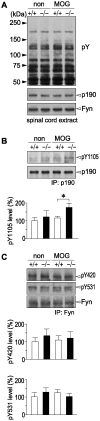
Background: Fyn tyrosine kinase-mediated down-regulation of Rho activity through activation of p190RhoGAP is crucial for oligodendrocyte differentiation and myelination. Therefore, the loss of function of its counterpart protein tyrosine phosphatase (PTP) may enhance myelination during development and remyelination in demyelinating diseases. To test this hypothesis, we investigated whether Ptprz, a receptor-like PTP (RPTP) expressed abuntantly in oligodendrocyte lineage cells, is involved in this process, because we recently revealed that p190RhoGAP is a physiological substrate for Ptprz.
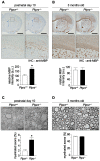
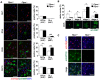
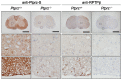
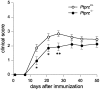
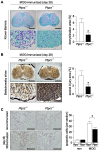
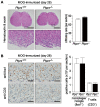
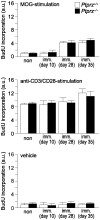
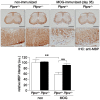
Methodology/principal findings: We found an early onset of the expression of myelin basic protein (MBP), a major protein of the myelin sheath, and early initiation of myelination in vivo during development of the Ptprz-deficient mouse, as compared with the wild-type. In addition, oligodendrocytes appeared earlier in primary cultures from Ptprz-deficient mice than wild-type mice. Furthermore, adult Ptprz-deficient mice were less susceptible to experimental autoimmune encephalomyelitis (EAE) induced by active immunization with myelin/oligodendrocyte glycoprotein (MOG) peptide than were wild-type mice. After EAE was induced, the tyrosine phosphorylation of p190RhoGAP increased significantly, and the EAE-induced loss of MBP was markedly suppressed in the white matter of the spinal cord in Ptprz-deficient mice. Here, the number of T-cells and macrophages/microglia infiltrating into the spinal cord did not differ between the two genotypes after MOG immunization. All these findings strongly support the validity of our hypothesis.
Conclusions/significance: Ptprz plays a negative role in oligodendrocyte differentiation in early central nervous system (CNS) development and remyelination in demyelinating CNS diseases, through the dephosphorylation of substrates such as p190RhoGAP.
Conflict of interest statement
Figures

Similar articles
-
Protein Tyrosine Phosphatase Receptor Type Z in Central Nervous System Disease.Int J Mol Sci. 2022 Apr 16;23(8):4414. doi: 10.3390/ijms23084414. Int J Mol Sci. 2022. PMID: 35457233 Free PMC article. Review.
-
Inactivation of Protein Tyrosine Phosphatase Receptor Type Z by Pleiotrophin Promotes Remyelination through Activation of Differentiation of Oligodendrocyte Precursor Cells.J Neurosci. 2015 Sep 2;35(35):12162-71. doi: 10.1523/JNEUROSCI.2127-15.2015. J Neurosci. 2015. PMID: 26338327 Free PMC article.
-
The PTN-PTPRZ signal activates the AFAP1L2-dependent PI3K-AKT pathway for oligodendrocyte differentiation: Targeted inactivation of PTPRZ activity in mice.Glia. 2019 May;67(5):967-984. doi: 10.1002/glia.23583. Epub 2019 Jan 22. Glia. 2019. PMID: 30667096
-
Role of Chondroitin Sulfate (CS) Modification in the Regulation of Protein-tyrosine Phosphatase Receptor Type Z (PTPRZ) Activity: PLEIOTROPHIN-PTPRZ-A SIGNALING IS INVOLVED IN OLIGODENDROCYTE DIFFERENTIATION.J Biol Chem. 2016 Aug 26;291(35):18117-28. doi: 10.1074/jbc.M116.742536. Epub 2016 Jul 21. J Biol Chem. 2016. PMID: 27445335 Free PMC article.
-
FGF/FGFR Pathways in Multiple Sclerosis and in Its Disease Models.Cells. 2021 Apr 13;10(4):884. doi: 10.3390/cells10040884. Cells. 2021. PMID: 33924474 Free PMC article. Review.
Cited by
-
Protein Tyrosine Phosphatase Receptor Type Z in Central Nervous System Disease.Int J Mol Sci. 2022 Apr 16;23(8):4414. doi: 10.3390/ijms23084414. Int J Mol Sci. 2022. PMID: 35457233 Free PMC article. Review.
-
Curcumin Modulates PTPRZ1 Activity and RNA m6A Modifications in Neuroinflammation-Associated Microglial Response.Adv Sci (Weinh). 2025 Apr;12(15):e2405263. doi: 10.1002/advs.202405263. Epub 2025 Feb 8. Adv Sci (Weinh). 2025. PMID: 39921492 Free PMC article.
-
Specific dephosphorylation at tyr-554 of git1 by ptprz promotes its association with paxillin and hic-5.PLoS One. 2015 Mar 5;10(3):e0119361. doi: 10.1371/journal.pone.0119361. eCollection 2015. PLoS One. 2015. PMID: 25742295 Free PMC article.
-
Glial Cells Promote Myelin Formation and Elimination.Front Cell Dev Biol. 2021 May 11;9:661486. doi: 10.3389/fcell.2021.661486. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34046407 Free PMC article. Review.
-
Comparative Analysis of Protein Tyrosine Phosphatases Regulating Microglial Activation.Exp Neurobiol. 2016 Oct;25(5):252-261. doi: 10.5607/en.2016.25.5.252. Epub 2016 Oct 20. Exp Neurobiol. 2016. PMID: 27790059 Free PMC article.
References
-
- Frohman EM, Racke MK, Raine CS (2006) Multiple sclerosis - the plaque and its pathogenesis. N Engl J Med 354: 942–955. - PubMed
-
- Scarlato M, Beesley J, Pleasure D (2000) Analysis of oligodendroglial differentiation using cDNA arrays. J Neurosci Res 59: 430–435. - PubMed
-
- Umemori H, Sato S, Yagi T, Aizawa S, Yamamoto T (1994) Initial events of myelination involve Fyn tyrosine kinase signalling. Nature 367: 572–576. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

