Generation and characterization of a defective HIV-1 Virus as an immunogen for a therapeutic vaccine
- PMID: 23144996
- PMCID: PMC3492255
- DOI: 10.1371/journal.pone.0048848
Generation and characterization of a defective HIV-1 Virus as an immunogen for a therapeutic vaccine
Abstract
Background: The generation of new immunogens able to elicit strong specific immune responses remains a major challenge in the attempts to obtain a prophylactic or therapeutic vaccine against HIV/AIDS. We designed and constructed a defective recombinant virus based on the HIV-1 genome generating infective but non-replicative virions able to elicit broad and strong cellular immune responses in HIV-1 seropositive individuals.
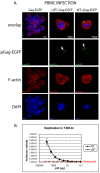
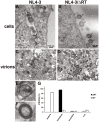
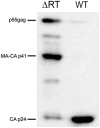
Results: Viral particles were generated through transient transfection in producer cells (293-T) of a full length HIV-1 DNA carrying a deletion of 892 base pairs (bp) in the pol gene encompassing the sequence that codes for the reverse transcriptase (NL4-3/ΔRT clone). The viral particles generated were able to enter target cells, but due to the absence of reverse transcriptase no replication was detected. The immunogenic capacity of these particles was assessed by ELISPOT to determine γ-interferon production in a cohort of 69 chronic asymptomatic HIV-1 seropositive individuals. Surprisingly, defective particles produced from NL4-3/ΔRT triggered stronger cellular responses than wild-type HIV-1 viruses inactivated with Aldrithiol-2 (AT-2) and in a larger proportion of individuals (55% versus 23% seropositive individuals tested). Electron microscopy showed that NL4-3/ΔRT virions display immature morphology. Interestingly, wild-type viruses treated with Amprenavir (APV) to induce defective core maturation also induced stronger responses than the same viral particles generated in the absence of protease inhibitors.
Conclusions: We propose that immature HIV-1 virions generated from NL4-3/ΔRT viral clones may represent new prototypes of immunogens with a safer profile and stronger capacity to induce cellular immune responses than wild-type inactivated viral particles.
Conflict of interest statement
Figures

References
-
- Ross AL, Brave A, Scarlatti G, Manrique A, Buonaguro L (2010) Progress towards development of an HIV vaccine: report of the AIDS Vaccine 2009 Conference. Lancet Infect Dis 10: 305–316. - PubMed
-
- Hel Z, Nacsa J, Tryniszewska E, Tsai WP, Parks RW, et al. (2002) Containment of simian immunodeficiency virus infection in vaccinated macaques: correlation with the magnitude of virus-specific pre- and postchallenge CD4+ and CD8+ T cell responses. J Immunol 169: 4778–4787. - PubMed
-
- Letvin NL, Walker BD (2003) Immunopathogenesis and immunotherapy in AIDS virus infections. Nat Med 9: 861–866. - PubMed
-
- Libois A, Lopez A, Garcia F, Castro P, Maleno MJ, et al. (2006) Dynamics of T cells subsets and lymphoproliferative responses during structured treatment interruption cycles and after definitive interruption of HAART in early chronic HIV type-1-infected patients. AIDS Res Hum Retroviruses 22: 657–666. - PubMed

