Induced tauopathy in a novel 3D-culture model mediates neurodegenerative processes: a real-time study on biochips
- PMID: 23145103
- PMCID: PMC3492324
- DOI: 10.1371/journal.pone.0049150
Induced tauopathy in a novel 3D-culture model mediates neurodegenerative processes: a real-time study on biochips
Abstract
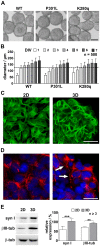
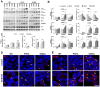
Tauopathies including Alzheimer's disease represent one of the major health problems of aging population worldwide. Therefore, a better understanding of tau-dependent pathologies and consequently, tau-related intervention strategies is highly demanded. In recent years, several tau-focused therapies have been proposed with the aim to stop disease progression. However, to develop efficient active pharmaceutical ingredients for the broad treatment of Alzheimer's disease patients, further improvements are necessary for understanding the detailed neurodegenerative processes as well as the mechanism and side effects of potential active pharmaceutical ingredients (API) in the neuronal system. In this context, there is a lack of suitable complex in vitro cell culture models recapitulating major aspects of taupathological degenerative processes in sufficient time and reproducible manner.Herewith, we describe a novel 3D SH-SY5Y cell-based, tauopathy model that shows advanced characteristics of matured neurons in comparison to monolayer cultures without the need of artificial differentiation promoting agents. Moreover, the recombinant expression of a novel highly pathologic fourfold mutated human tau variant lead to a fast and emphasized degeneration of neuritic processes. The neurodegenerative effects could be analyzed in real time and with high sensitivity using our unique microcavity array-based impedance spectroscopy measurement system. We were able to quantify a time- and concentration-dependent relative impedance decrease when Alzheimer's disease-like tau pathology was induced in the neuronal 3D cell culture model. In combination with the collected optical information, the degenerative processes within each 3D-culture could be monitored and analyzed. More strikingly, tau-specific regenerative effects caused by tau-focused active pharmaceutical ingredients could be quantitatively monitored by impedance spectroscopy.Bringing together our novel complex 3D cell culture taupathology model and our microcavity array-based impedimetric measurement system, we provide a powerful tool for the label-free investigation of tau-related pathology processes as well as the high content analysis of potential active pharmaceutical ingredient candidates.
Conflict of interest statement
Figures



Comment in
-
Neurodegenerative disease: a novel 3D culture model of tauopathy shows promise as a screening tool for Alzheimer disease therapies.Nat Rev Neurol. 2013 Jan;9(1):2. doi: 10.1038/nrneurol.2012.245. Epub 2012 Dec 4. Nat Rev Neurol. 2013. PMID: 23208115 No abstract available.
References
-
- Rodgers AB (2008) ALZHEIMER’S DISEASE Unraveling the Mystery. In: Aging NIoHNIo, editor. Silver Spring. 79.
-
- Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM (2007) Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement 3: 186–191. - PubMed
-
- Ittner LM, Gotz J (2011) Amyloid-beta and tau–a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci 12: 65–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

