Induction of brain microvascular endothelial cell urokinase expression by Cryptococcus neoformans facilitates blood-brain barrier invasion
- PMID: 23145170
- PMCID: PMC3493525
- DOI: 10.1371/journal.pone.0049402
Induction of brain microvascular endothelial cell urokinase expression by Cryptococcus neoformans facilitates blood-brain barrier invasion
Abstract
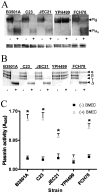
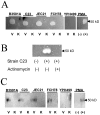
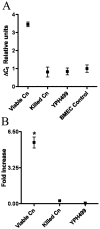
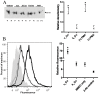
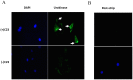
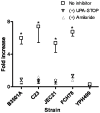
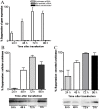
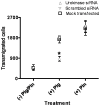
The invasive ability of the blood-borne fungal pathogen Cryptococcus neoformans can be enhanced through interactions with host plasma components, such as plasminogen. Previously we showed by in vitro studies that plasminogen coats the surface of C. neoformans and is converted to the active serine protease, plasmin, by host plasminogen activators. Viable, but not formaldehyde- or sodium azide-killed, cryptococcal strains undergo brain microvascular endothelial cell-dependent plasminogen-to-plasmin activation, which results in enhanced, plasmin-dependent cryptococcal invasion of primary bovine brain microvascular endothelial cells and fungal ability to degrade plasmin substrates. In the present work, brain microvascular endothelial cells cultured with viable, but not killed, cryptococcal strains led to significant increases in both urokinase mRNA transcription and cell-associated urokinase protein expression. Soluble urokinase was also detected in conditioned medium from brain microvascular endothelial cells cultured with viable, but not killed, C. neoformans. Exposure of plasminogen pre-coated viable C. neoformans to conditioned medium from strain-matched brain microvascular endothelial cell-fungal co-cultures resulted in plasminogen-to-plasmin activation and plasmin-dependent cryptococcal invasion. siRNA-mediated silencing of urokinase gene expression or the use of specific inhibitors of urokinase activity abrogated both plasminogen-to-plasmin activation on C. neoformans and cryptococcal-brain microvascular endothelial cell invasion. Our results suggest that pathogen exploitation of the host urokinase-plasmin(ogen) system may contribute to C. neoformans virulence during invasive cryptococcosis.
Conflict of interest statement
Figures

Similar articles
-
Blood-brain barrier invasion by Cryptococcus neoformans is enhanced by functional interactions with plasmin.Microbiology (Reading). 2012 Jan;158(Pt 1):240-258. doi: 10.1099/mic.0.051524-0. Epub 2011 Oct 13. Microbiology (Reading). 2012. PMID: 21998162 Free PMC article.
-
Group B Streptococcus hijacks the host plasminogen system to promote brain endothelial cell invasion.PLoS One. 2013 May 2;8(5):e63244. doi: 10.1371/journal.pone.0063244. Print 2013. PLoS One. 2013. PMID: 23658816 Free PMC article.
-
Cryptococcus neoformans activates RhoGTPase proteins followed by protein kinase C, focal adhesion kinase, and ezrin to promote traversal across the blood-brain barrier.J Biol Chem. 2012 Oct 19;287(43):36147-57. doi: 10.1074/jbc.M112.389676. Epub 2012 Aug 16. J Biol Chem. 2012. PMID: 22898813 Free PMC article.
-
How Cryptococcus interacts with the blood-brain barrier.Future Microbiol. 2015;10(10):1669-82. doi: 10.2217/fmb.15.83. Epub 2015 Oct 6. Future Microbiol. 2015. PMID: 26437710 Review.
-
Cryptococcus neoformans-astrocyte interactions: effect on fungal blood brain barrier disruption, brain invasion, and meningitis progression.Crit Rev Microbiol. 2021 Mar;47(2):206-223. doi: 10.1080/1040841X.2020.1869178. Epub 2021 Jan 21. Crit Rev Microbiol. 2021. PMID: 33476528 Free PMC article. Review.
Cited by
-
The pathways and the mechanisms by which Cryptococcus enters the brain.Mycology. 2024 Feb 14;15(3):345-359. doi: 10.1080/21501203.2023.2295409. eCollection 2024. Mycology. 2024. PMID: 39247889 Free PMC article. Review.
-
A critical role for plasminogen in inflammation.J Exp Med. 2020 Apr 6;217(4):e20191865. doi: 10.1084/jem.20191865. J Exp Med. 2020. PMID: 32159743 Free PMC article. Review.
-
From the blood to the brain: avenues of eukaryotic pathogen dissemination to the central nervous system.Curr Opin Microbiol. 2015 Aug;26:53-9. doi: 10.1016/j.mib.2015.05.006. Epub 2015 Jun 3. Curr Opin Microbiol. 2015. PMID: 26048316 Free PMC article. Review.
-
Plasminogen Activators in Neurovascular and Neurodegenerative Disorders.Int J Mol Sci. 2021 Apr 22;22(9):4380. doi: 10.3390/ijms22094380. Int J Mol Sci. 2021. PMID: 33922229 Free PMC article. Review.
-
Virulence of Cryptococcus sp. Biofilms In Vitro and In Vivo using Galleria mellonella as an Alternative Model.Front Microbiol. 2016 Mar 9;7:290. doi: 10.3389/fmicb.2016.00290. eCollection 2016. Front Microbiol. 2016. PMID: 27014214 Free PMC article.
References
-
- Bennett JE, Kwon-Chung KJ, Howard DH (1977) Epidemiologic differences among serotypes of Cryptococcus neoformans . Am J Epidemiol 105: 582–586. - PubMed
-
- Kwon-Chung KJ, Bennett JE (1984) Epidemiologic differences between the two varieties of Cryptococcus neoformans . Am J Epidemiol 120: 123–130. - PubMed
-
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, et al. (2009) Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23: 525–530. - PubMed
-
- Powderly WG (1993) Cryptococcal meningitis and AIDS. Clin Infect Dis 17: 837–842. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

