Carboxylesterase1/Esterase-x regulates chylomicron production in mice
- PMID: 23145182
- PMCID: PMC3492262
- DOI: 10.1371/journal.pone.0049515
Carboxylesterase1/Esterase-x regulates chylomicron production in mice
Abstract
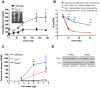
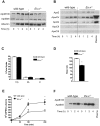
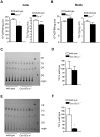
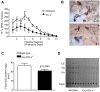
Elevated postprandial plasma triacylglycerol (TG) concentrations are commonly associated with obesity and the risk of cardiovascular disease. Dietary fat contributes to this condition through the production of chylomicrons. Carboxylesterases have been mainly studied for their role in drug metabolism, but recently they have been shown to participate in lipid metabolism; however, their role in intestinal lipid metabolism is unknown. Carboxylesterase1/esterase-x (Ces1/Es-x) deficient mice become obese, hyperlipidemic and develop hepatic steatosis even on standard chow diet. Here, we aimed to explore the role of Ces1/Es-x in intestinal lipid metabolism. Six-month old wild-type and Ces1/Es-x deficient mice were maintained on chow diet and intestinal lipid metabolism and plasma chylomicron clearance were analyzed. Along the intestine Ces1/Es-x protein is expressed only in proximal jejunum. Ablation of Ces1/Es-x expression results in postprandial hyperlipidemia due to increased secretion of chylomicrons. The secreted chylomicrons have aberrant protein composition, which results in their reduced clearance. In conclusion, Ces1/Es-x participates in the regulation of chylomicron assembly and secretion. Ces1/Es-x might act as a lipid sensor in enterocytes regulating chylomicron secretion rate. Ces1/Es-x might represent an attractive pharmacological target for the treatment of lipid abnormalities associated with obesity, insulin resistance and fatty liver disease.
Conflict of interest statement
Figures

References
-
- Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A (2007) Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 298: 299–308. - PubMed
-
- Quiroga AD, Li L, Trotzmuller M, Nelson R, Proctor SD, et al.. (2012) Deficiency of carboxylesterase 1/esterase-x in mice results in obesity, hepatic steatosis and hyperlipidemia. Hepatology. doi: 10.1002/hep.25961. - PubMed
-
- Wei E, Ben Ali Y, Lyon J, Wang H, Nelson R, et al. (2010) Loss of TGH/Ces3 in mice decreases blood lipids, improves glucose tolerance, and increases energy expenditure. Cell Metab 11: 183–193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

