P2Y receptors and kidney function
- PMID: 23145369
- PMCID: PMC3490424
- DOI: 10.1002/wmts.61
P2Y receptors and kidney function
Abstract
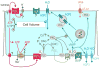
Cellular release of nucleotides is of physiological importance to regulate and maintain cell function and integrity. Also in the tubular and collecting duct system of the kidney, nucleotides are released in response to changes in cell volume or luminal flow rate and act in a paracrine and autocrine way on basolateral and luminal P2Y receptors. Recent studies using gene knockout mice assigned a prominent role to G protein-coupled P2Y(2) receptors, which are activated by both ATP and UTP. The antidiuretic hormone, arginine-vasopressin (AVP), and possibly an increase in collecting duct cell volume induce ATP release. The subsequent activation of P2Y(2) receptors inhibits AVP-induced cAMP formation and water reabsorption, which stabilizes cell volume and facilitates water excretion. An increase in NaCl intake enhances luminal release of ATP and UTP in the aldosterone-sensitive distal nephron which by activating apical P2Y(2) receptors and phospholipase C lowers the open probability of the epithelial sodium channel ENaC, thereby facilitating sodium excretion. Thus, the renal ATP/UTP/P2Y(2) receptor system not only serves to preserve cell volume and integrity but is also regulated by stimuli that derive from body NaCl homeostasis. The system also inhibits ENaC activity during aldosterone escape, i.e. when sodium reabsorption via ENaC is inappropriately high. The P2Y(2) receptor tone inhibits the expression and activity of the Na-K-2Cl cotransporter NKCC2 in the thick ascending limb and mediates vasodilation. While the role of other P2Y receptors in the kidney is less clear, the ATP/UTP/P2Y(2) receptor system regulates NaCl and water homeostasis and blood pressure.
Figures




Similar articles
-
Regulation of renal NaCl and water transport by the ATP/UTP/P2Y2 receptor system.Am J Physiol Renal Physiol. 2011 Sep;301(3):F463-75. doi: 10.1152/ajprenal.00236.2011. Epub 2011 Jun 29. Am J Physiol Renal Physiol. 2011. PMID: 21715471 Free PMC article. Review.
-
Dietary Na+ inhibits the open probability of the epithelial sodium channel in the kidney by enhancing apical P2Y2-receptor tone.FASEB J. 2010 Jun;24(6):2056-65. doi: 10.1096/fj.09-151506. Epub 2010 Jan 22. FASEB J. 2010. PMID: 20097874 Free PMC article.
-
Control of epithelial transport via luminal P2 receptors.Am J Physiol Renal Physiol. 2003 Mar;284(3):F419-32. doi: 10.1152/ajprenal.00075.2002. Am J Physiol Renal Physiol. 2003. PMID: 12556361 Review.
-
Renal sodium transporter/channel expression and sodium excretion in P2Y2 receptor knockout mice fed a high-NaCl diet with/without aldosterone infusion.Am J Physiol Renal Physiol. 2011 Mar;300(3):F657-68. doi: 10.1152/ajprenal.00549.2010. Epub 2010 Dec 29. Am J Physiol Renal Physiol. 2011. PMID: 21190950 Free PMC article.
-
Distal colonic Na(+) absorption inhibited by luminal P2Y(2) receptors.Pflugers Arch. 2007 Sep;454(6):977-87. doi: 10.1007/s00424-007-0248-9. Epub 2007 Mar 14. Pflugers Arch. 2007. PMID: 17356885
Cited by
-
Purinergic signaling in the male reproductive tract.Front Endocrinol (Lausanne). 2022 Nov 7;13:1049511. doi: 10.3389/fendo.2022.1049511. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36419764 Free PMC article. Review.
-
Interplay between renal endothelin and purinergic signaling systems.Am J Physiol Renal Physiol. 2017 Sep 1;313(3):F666-F668. doi: 10.1152/ajprenal.00639.2016. Epub 2017 Feb 8. Am J Physiol Renal Physiol. 2017. PMID: 28179257 Free PMC article. Review.
-
Emerging key roles for P2X receptors in the kidney.Front Physiol. 2013 Sep 27;4:262. doi: 10.3389/fphys.2013.00262. eCollection 2013. Front Physiol. 2013. PMID: 24098285 Free PMC article.
-
P2Y2 receptor decreases blood pressure by inhibiting ENaC.JCI Insight. 2023 Jul 24;8(14):e167704. doi: 10.1172/jci.insight.167704. JCI Insight. 2023. PMID: 37279066 Free PMC article.
-
Pannexin 1 channels in renin-expressing cells influence renin secretion and blood pressure homeostasis.Kidney Int. 2020 Sep;98(3):630-644. doi: 10.1016/j.kint.2020.04.041. Epub 2020 May 21. Kidney Int. 2020. PMID: 32446934 Free PMC article.
References
-
- Vallon V. P2 receptors in the regulation of renal transport mechanisms. Am J Physiol Renal Physiol. 2008;294:F10–F27. - PubMed
-
- Praetorius HA, Leipziger J. Intrarenal purinergic signaling in the control of renal tubular transport. Annu Rev Physiol. 2010;72:377–393. - PubMed
-
- Schwiebert EM. ATP release mechanisms, ATP receptors and purinergic signalling along the nephron. Clin Exp Pharmacol Physiol. 2001;28:340–350. - PubMed
-
- Vekaria RM, Shirley DG, Sevigny J, Unwin RJ. Immunolocalization of ectonucleotidases along the rat nephron. Am J Physiol Renal Physiol. 2006;290:F550–F560. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
