A randomized trial of tigecycline versus ampicillin-sulbactam or amoxicillin-clavulanate for the treatment of complicated skin and skin structure infections
- PMID: 23145952
- PMCID: PMC3560230
- DOI: 10.1186/1471-2334-12-297
A randomized trial of tigecycline versus ampicillin-sulbactam or amoxicillin-clavulanate for the treatment of complicated skin and skin structure infections
Abstract
Background: Complicated skin and skin structure infections (cSSSIs) frequently result in hospitalization with significant morbidity and mortality.
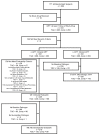
Methods: In this phase 3b/4 parallel, randomized, open-label, comparative study, 531 subjects with cSSSI received tigecycline (100 mg initial dose, then 50 mg intravenously every 12 hrs) or ampicillin-sulbactam 1.5-3 g IV every 6 hrs or amoxicillin-clavulanate 1.2 g IV every 6-8 hrs. Vancomycin could be added at the discretion of the investigator to the comparator arm if methicillin-resistant Staphylococcus aureus (MRSA) was confirmed or suspected within 72 hrs of enrollment. The primary endpoint was clinical response in the clinically evaluable (CE) population at the test-of-cure (TOC) visit. Microbiologic response and safety were also assessed. The modified intent-to-treat (mITT) population comprised 531 subjects (tigecycline, n = 268; comparator, n = 263) and 405 were clinically evaluable (tigecycline, n = 209; comparator, n = 196).
Results: In the CE population, 162/209 (77.5%) tigecycline-treated subjects and 152/196 (77.6%) comparator-treated subjects were clinically cured (difference 0.0; 95% confidence interval [CI]: -8.7, 8.6). The eradication rates at the subject level for the microbiologically evaluable (ME) population were 79.2% in the tigecycline treatment group and 76.8% in the comparator treatment group (difference 2.4; 95% CI: -9.6, 14.4) at the TOC assessment. Nausea, vomiting, and diarrhea rates were higher in the tigecycline group.
Conclusions: Tigecycline was generally safe and effective in the treatment of cSSSIs.
Trial registration: ClinicalTrials.gov NCT00368537.
Figures
References
-
- US Department of Health and Human Services Food and Drug Administration. Guidance for industry. Acute bacterial skin and skin structure infections: developing drugs for treatment. Draft guidance. U.S. Department of Health and Human Services, Washington, D.C; 2010. http://www.fda.gov/downloads/Drugs/../Guidances/ucm071185.pdf.
-
- Jones ME, Karlowsky JA, Draghi DC, Thornsberry C, Sahm DF, Nathwani D. Epidemiology and antibiotic susceptibility of bacteria causing skin and soft tissue infections in the USA and Europe: a guide to appropriate antimicrobial therapy. Int J Antimicrob Agents. 2003;22:406–419. doi: 10.1016/S0924-8579(03)00154-7. - DOI - PubMed
-
- Moet GJ, Jones RN, Biedenbach DJ, Stilwell MG, Fritsche TR. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY Antimicrobial Surveillance Program (1998-2004) Diagnostic Microbiol Infect Dis. 2007;57:7–13. doi: 10.1016/j.diagmicrobio.2006.05.009. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

