Polarity and cell fate asymmetry in Caulobacter crescentus
- PMID: 23146566
- PMCID: PMC3587792
- DOI: 10.1016/j.mib.2012.10.011
Polarity and cell fate asymmetry in Caulobacter crescentus
Abstract
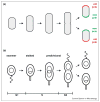
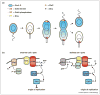
The production of asymmetric daughter cells is a hallmark of metazoan development and critical to the life cycle of many microbes, including the α-proteobacterium Caulobacter crescentus. For Caulobacter, every cell division is asymmetric, yielding daughter cells with different morphologies and replicative potentials. This asymmetry in daughter cell fate is governed by the response regulator CtrA, a transcription factor that can also bind and silence the origin of replication. CtrA activity is controlled by a complex regulatory circuit that includes several polarly localized histidine kinases. This circuit ensures differential activation of CtrA in daughter cells, leading to their asymmetric replicative potentials. Here, we review progress in elucidating the molecular mechanisms regulating CtrA and the role of cellular polarity in this process.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
A genetic oscillator and the regulation of cell cycle progression in Caulobacter crescentus.Cell Cycle. 2004 Oct;3(10):1252-4. doi: 10.4161/cc.3.10.1181. Epub 2004 Oct 17. Cell Cycle. 2004. PMID: 15467452 Review.
-
A dynamic complex of signaling proteins uses polar localization to regulate cell-fate asymmetry in Caulobacter crescentus.Dev Cell. 2011 Mar 15;20(3):329-41. doi: 10.1016/j.devcel.2011.01.007. Dev Cell. 2011. PMID: 21397844 Free PMC article.
-
Selective sequestration of signalling proteins in a membraneless organelle reinforces the spatial regulation of asymmetry in Caulobacter crescentus.Nat Microbiol. 2020 Mar;5(3):418-429. doi: 10.1038/s41564-019-0647-7. Epub 2020 Jan 20. Nat Microbiol. 2020. PMID: 31959967 Free PMC article.
-
Dynamics of two Phosphorelays controlling cell cycle progression in Caulobacter crescentus.J Bacteriol. 2009 Dec;191(24):7417-29. doi: 10.1128/JB.00992-09. Epub 2009 Sep 25. J Bacteriol. 2009. PMID: 19783630 Free PMC article.
-
Spatial and temporal control of differentiation and cell cycle progression in Caulobacter crescentus.Annu Rev Microbiol. 2003;57:225-47. doi: 10.1146/annurev.micro.57.030502.091006. Annu Rev Microbiol. 2003. PMID: 14527278 Review.
Cited by
-
Regulated proteolysis of a transcription factor complex is critical to cell cycle progression in Caulobacter crescentus.Mol Microbiol. 2013 Mar;87(6):1277-89. doi: 10.1111/mmi.12166. Epub 2013 Feb 25. Mol Microbiol. 2013. PMID: 23368090 Free PMC article.
-
The Caulobacter crescentus DciA promotes chromosome replication through topological loading of the DnaB replicative helicase at replication forks.Nucleic Acids Res. 2022 Dec 9;50(22):12896-12912. doi: 10.1093/nar/gkac1146. Nucleic Acids Res. 2022. PMID: 36484102 Free PMC article.
-
Age structure landscapes emerge from the equilibrium between aging and rejuvenation in bacterial populations.Nat Commun. 2018 Sep 13;9(1):3722. doi: 10.1038/s41467-018-06154-9. Nat Commun. 2018. PMID: 30213942 Free PMC article.
-
Fifty years after the replicon hypothesis: cell-specific master regulators as new players in chromosome replication control.J Bacteriol. 2014 Aug 15;196(16):2901-11. doi: 10.1128/JB.01706-14. Epub 2014 Jun 9. J Bacteriol. 2014. PMID: 24914187 Free PMC article. Review.
-
Short-Stalked Prosthecomicrobium hirschii Cells Have a Caulobacter-Like Cell Cycle.J Bacteriol. 2016 Feb 1;198(7):1149-59. doi: 10.1128/JB.00896-15. J Bacteriol. 2016. PMID: 26833409 Free PMC article.
References
-
- Quon KC, Marczynski GT, Shapiro L. Cell cycle control by an essential bacterial two-component signal transduction protein. Cell. 1996;84:83–93. Reported discovery of CtrA as an essential response regulator and cell cycle transcription factor. - PubMed
-
- Laub MT, McAdams HH, Feldblyum T, Fraser CM, Shapiro L. Global analysis of the genetic network controlling a bacterial cell cycle. Science. 2000;290:2144–2148. - PubMed
-
- Quon KC, Yang B, Domian IJ, Shapiro L, Marczynski GT. Negative control of bacterial DNA replication by a cell cycle regulatory protein that binds at the chromosome origin. Proc Natl Acad Sci USA. 1998;95:120–125. Demonstrated that CtrA directly binds and silences the origin of replication. - PMC - PubMed
-
- Bastedo DP, Marczynski GT. CtrA response regulator binding to the Caulobacter chromosome replication origin is required during nutrient and antibiotic stress as well as during cell cycle progression. Mol Microbiol. 2009;72:139–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

