A full-length recombinant Plasmodium falciparum PfRH5 protein induces inhibitory antibodies that are effective across common PfRH5 genetic variants
- PMID: 23146673
- PMCID: PMC3538003
- DOI: 10.1016/j.vaccine.2012.10.106
A full-length recombinant Plasmodium falciparum PfRH5 protein induces inhibitory antibodies that are effective across common PfRH5 genetic variants
Abstract
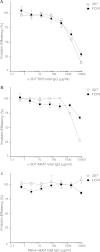
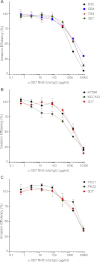
The lack of an effective licensed vaccine remains one of the most significant gaps in the portfolio of tools being developed to eliminate Plasmodium falciparum malaria. Vaccines targeting erythrocyte invasion - an essential step for both parasite development and malaria pathogenesis - have faced the particular challenge of genetic diversity. Immunity-driven balancing selection pressure on parasite invasion proteins often results in the presence of multiple, antigenically distinct, variants within a population, leading to variant-specific immune responses. Such variation makes it difficult to design a vaccine that covers the full range of diversity, and could potentially facilitate the evolution of vaccine-resistant parasite strains. In this study, we investigate the effect of genetic diversity on invasion inhibition by antibodies to a high priority P. falciparum invasion candidate antigen, P. falciparum Reticulocyte Binding Protein Homologue 5 (PfRH5). Previous work has shown that virally delivered PfRH5 can induce antibodies that protect against a wide range of genetic variants. Here, we show that a full-length recombinant PfRH5 protein expressed in mammalian cells is biochemically active, as judged by saturable binding to its receptor, basigin, and is able to induce antibodies that strongly inhibit P. falciparum growth and invasion. Whole genome sequencing of 290 clinical P. falciparum isolates from across the world identifies only five non-synonymous PfRH5 SNPs that are present at frequencies of 10% or more in at least one geographical region. Antibodies raised against the 3D7 variant of PfRH5 were able to inhibit nine different P. falciparum strains, which between them included all of the five most common PfRH5 SNPs in this dataset, with no evidence for strain-specific immunity. We conclude that protein-based PfRH5 vaccines are an urgent priority for human efficacy trials.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures


References
-
- Vaughan A.M., Kappe S.H. Malaria vaccine development: persistent challenges. Curr Opin Immunol. 2012;April:20. - PubMed
-
- Butler N.S., Vaughan A.M., Harty J.T., Kappe S.H. Whole parasite vaccination approaches for prevention of malaria infection. Trends Immunol. 2012;33(May (5)):247–254. - PubMed
-
- Coppel R.L. Vaccinating with the genome: a Sisyphean task? Trends Parasitol. 2009;25(May (5)):205–212. - PubMed
-
- Cohen S., Mc G.I., Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192(November):733–737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

