Pharmacokinetics of single ascending doses of the P-glycoprotein inhibitor tariquidar in healthy subjects
- PMID: 23146816
- PMCID: PMC3689924
- DOI: 10.1159/000343243
Pharmacokinetics of single ascending doses of the P-glycoprotein inhibitor tariquidar in healthy subjects
Abstract
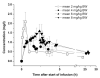
We assessed the pharmacokinetics (PK), tolerability and safety of tariquidar (TQD), a P-glycoprotein (Pgp) inhibitor, after intravenous administration of single ascending doses. Employed doses were up to 4-fold higher than in previous clinical trials in cancer patients and are capable of inhibiting Pgp at the blood-brain barrier. Fifteen male healthy volunteers were randomized to receive single intravenous doses of TQD at 4, 6 or 8 mg/kg body weight and underwent blood sampling for over 24 h. TQD concentrations were determined in plasma samples with high-performance liquid chromatography mass spectrometry. No dose-limiting toxicities of TQD were observed. The area under the plasma concentration-time curve from start until 24 h after the end of infusion was positively correlated with an administered TQD dose (r = 0.8981, p < 0.0001). Moreover, we found a positive correlation for volume of distribution at steady state (r = 0.7129, p = 0.0004) with TQD dose. Dose dependency of volume of distribution at steady state points to non-linear PK of TQD, which was in all likelihood caused by transporter saturation at high TQD doses. Acceptable safety and tolerability as well as dose-linear increases in plasma exposure support the future use of TQD at doses up to 8 mg/kg to inhibit Pgp at the human blood-brain barrier.
Copyright © 2012 S. Karger AG, Basel.
Figures

Similar articles
-
Tolerability of tariquidar - A third generation P-gp inhibitor as add-on medication to antiseizure medications in drug-resistant epilepsy.Seizure. 2024 Jul;119:44-51. doi: 10.1016/j.seizure.2024.05.007. Epub 2024 May 15. Seizure. 2024. PMID: 38776617
-
Tariquidar and elacridar are dose-dependently transported by P-glycoprotein and Bcrp at the blood-brain barrier: a small-animal positron emission tomography and in vitro study.Drug Metab Dispos. 2013 Apr;41(4):754-62. doi: 10.1124/dmd.112.049148. Epub 2013 Jan 10. Drug Metab Dispos. 2013. PMID: 23305710
-
Increased permeability-glycoprotein inhibition at the human blood-brain barrier can be safely achieved by performing PET during peak plasma concentrations of tariquidar.J Nucl Med. 2015 Jan;56(1):82-7. doi: 10.2967/jnumed.114.146894. Epub 2014 Dec 11. J Nucl Med. 2015. PMID: 25500831 Free PMC article. Clinical Trial.
-
Uptake and binding of the serotonin 5-HT1A antagonist [18F]-MPPF in brain of rats: effects of the novel P-glycoprotein inhibitor tariquidar.Neuroimage. 2010 Jan 15;49(2):1406-15. doi: 10.1016/j.neuroimage.2009.09.048. Epub 2009 Sep 28. Neuroimage. 2010. PMID: 19796699
-
Modulation and prevention of multidrug resistance by inhibitors of P-glycoprotein.Cancer Chemother Pharmacol. 1997;40 Suppl:S13-9. doi: 10.1007/s002800051055. Cancer Chemother Pharmacol. 1997. PMID: 9272128 Review.
Cited by
-
Effects of Phytochemical P-Glycoprotein Modulators on the Pharmacokinetics and Tissue Distribution of Doxorubicin in Mice.Molecules. 2018 Feb 7;23(2):349. doi: 10.3390/molecules23020349. Molecules. 2018. PMID: 29414892 Free PMC article.
-
Effect of P-glycoprotein inhibition at the blood-brain barrier on brain distribution of (R)-[11 C]verapamil in elderly vs. young subjects.Br J Clin Pharmacol. 2017 Sep;83(9):1991-1999. doi: 10.1111/bcp.13301. Epub 2017 May 4. Br J Clin Pharmacol. 2017. PMID: 28401570 Free PMC article. Clinical Trial.
-
Simultaneous quantification of ondansetron and tariquidar in rat and human plasma using a high performance liquid chromatography-ultraviolet method.Biomed Chromatogr. 2019 Nov;33(11):e4653. doi: 10.1002/bmc.4653. Epub 2019 Sep 1. Biomed Chromatogr. 2019. PMID: 31322284 Free PMC article.
-
Pharmacokinetic and pharmacodynamic study of tariquidar (XR9576), a P-glycoprotein inhibitor, in combination with doxorubicin, vinorelbine, or docetaxel in children and adolescents with refractory solid tumors.Cancer Chemother Pharmacol. 2015 Dec;76(6):1273-83. doi: 10.1007/s00280-015-2845-1. Epub 2015 Oct 20. Cancer Chemother Pharmacol. 2015. PMID: 26486517 Free PMC article. Clinical Trial.
-
15-0600-Dr. Fox's response to letter to the editor.Cancer Chemother Pharmacol. 2016 Nov;78(5):1099-1100. doi: 10.1007/s00280-016-3162-z. Epub 2016 Oct 11. Cancer Chemother Pharmacol. 2016. PMID: 27730303 Free PMC article. No abstract available.
References
-
- Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–236. - PMC - PubMed
-
- Tiwari AK, Sodani K, Dai CL, Ashby CR, Jr., Chen ZS. Revisiting the ABCs of multidrug resistance in cancer chemotherapy. Curr Pharm Biotechnol. 2011;12:570–594. - PubMed
-
- Löscher W, Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci. 2005;6:591–602. - PubMed
-
- Fox E, Bates SE. Tariquidar (XR9576): a P-glycoprotein drug efflux pump inhibitor. Expert Rev Anticancer Ther. 2007;7:447–459. - PubMed
-
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

