Alveolar epithelial cells are critical in protection of the respiratory tract by secretion of factors able to modulate the activity of pulmonary macrophages and directly control bacterial growth
- PMID: 23147039
- PMCID: PMC3536158
- DOI: 10.1128/IAI.00950-12
Alveolar epithelial cells are critical in protection of the respiratory tract by secretion of factors able to modulate the activity of pulmonary macrophages and directly control bacterial growth
Abstract
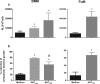
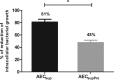
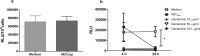
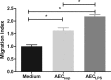
The respiratory epithelium is a physical and functional barrier actively involved in the clearance of environmental agents. The alveolar compartment is lined with membranous pneumocytes, known as type I alveolar epithelial cells (AEC I), and granular pneumocytes, type II alveolar epithelial cells (AEC II). AEC II are responsible for epithelial reparation upon injury and ion transport and are very active immunologically, contributing to lung defense by secreting antimicrobial factors. AEC II also secrete a broad variety of factors, such as cytokines and chemokines, involved in activation and differentiation of immune cells and are able to present antigen to specific T cells. Another cell type important in lung defense is the pulmonary macrophage (PuM). Considering the architecture of the alveoli, a good communication between the external and the internal compartments is crucial to mount effective responses. Our hypothesis is that being in the interface, AEC may play an important role in transmitting signals from the external to the internal compartment and in modulating the activity of PuM. For this, we collected supernatants from AEC unstimulated or stimulated in vitro with lipopolysaccharide (LPS). These AEC-conditioned media were used in various setups to test for the effects on a number of macrophage functions: (i) migration, (ii) phagocytosis and intracellular control of bacterial growth, and (iii) phenotypic changes and morphology. Finally, we tested the direct effect of AEC-conditioned media on bacterial growth. We found that AEC-secreted factors had a dual effect, on one hand controlling bacterial growth and on the other hand increasing macrophage activity.
Figures
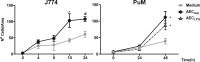
References
-
- Ryu JH, Kim CH, Yoon JH. 2010. Innate immune responses of the airway epithelium. Mol. Cells 30:173–183 - PubMed
-
- Williams MC. 2003. Alveolar type I cells: molecular phenotype and development. Annu. Rev. Physiol. 65:669–695 - PubMed
-
- Fereol S, Fodil R, Pelle G, Louis B, Isabey D. 2008. Cell mechanics of alveolar epithelial cells (AECs) and macrophages (AMs). Respir. Physiol. Neurobiol. 163:3–16 - PubMed
-
- Castranova V, Rabovsky J, Tucker JH, Miles PR. 1988. The alveolar type II epithelial cell: a multifunctional pneumocyte. Toxicol. Appl. Pharmacol. 93:472–483 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

