A rationally engineered anti-HIV peptide fusion inhibitor with greatly reduced immunogenicity
- PMID: 23147734
- PMCID: PMC3553736
- DOI: 10.1128/AAC.01152-12
A rationally engineered anti-HIV peptide fusion inhibitor with greatly reduced immunogenicity
Abstract
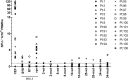
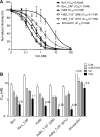
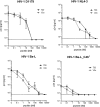
Peptides derived from the C-terminal heptad repeat 2 (HR2) region of the HIV-1 gp41 envelope glycoprotein, so-called C peptides, are very efficient HIV-1 fusion inhibitors. We previously developed innovative gene therapeutic approaches aiming at the direct in vivo production of C peptides from genetically modified host cells and found that T cells expressing membrane-anchored or secreted C peptides are protected from HIV-1 infection. However, an unwanted immune response against such antiviral peptides may significantly impair clinical efficacy and pose safety risks to patients. To overcome this problem, we engineered a novel C peptide, V2o, with greatly reduced immunogenicity and excellent antiviral activity. V2o is based on the chimeric C peptide C46-EHO, which is derived from the HR2 regions of HIV-2(EHO) and HIV-1(HxB2) and has broad anti-HIV and anti-simian immunodeficiency virus activity. Antibody and major histocompatibility complex class I epitopes within the C46-EHO peptide sequence were identified by in silico and in vitro analyses. Using rational design, we removed these epitopes by amino acid substitutions and thus minimized antigenicity and immunogenicity considerably. At the same time, the antiviral activity of the deimmunized peptide V2o was preserved or even enhanced compared to that of the parental C46-EHO peptide. Thus, V2o is an ideal candidate, especially for those novel therapeutic approaches for HIV infection that involve direct in vivo production of antiviral C peptides.
Figures


References
-
- Lalezari JP, Eron JJ, Carlson M, Cohen C, DeJesus E, Arduino RC, Gallant JE, Volberding P, Murphy RL, Valentine F, Nelson EL, Sista PR, Dusek A, Kilby JM. 2003. A phase II clinical study of the long-term safety and antiviral activity of enfuvirtide-based antiretroviral therapy. AIDS 17:691–698 - PubMed
-
- Rammensee HG, Friede T, Stevanoviic S. 1995. MHC ligands and peptide motifs: first listing. Immunogenetics 41:178–228 - PubMed
-
- Reche PA, Reinherz EL. 2003. Sequence variability analysis of human class I and class II MHC molecules: functional and structural correlates of amino acid polymorphisms. J. Mol. Biol. 331:623–641 - PubMed
-
- Falk K, Rotzschke O, Stevanovic S, Jung G, Rammensee HG. 1991. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature 351:290–296 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

