Novel carbapenem antibiotics for parenteral and oral applications: in vitro and in vivo activities of 2-aryl carbapenems and their pharmacokinetics in laboratory animals
- PMID: 23147735
- PMCID: PMC3553697
- DOI: 10.1128/AAC.01051-12
Novel carbapenem antibiotics for parenteral and oral applications: in vitro and in vivo activities of 2-aryl carbapenems and their pharmacokinetics in laboratory animals
Abstract
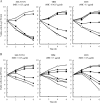
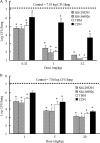
SM-295291 and SM-369926 are new parenteral 2-aryl carbapenems with strong activity against major causative pathogens of community-acquired infections such as methicillin-susceptible Staphylococcus aureus, Streptococcus pneumoniae (including penicillin-resistant strains), Streptococcus pyogenes, Enterococcus faecalis, Klebsiella pneumoniae, Moraxella catarrhalis, Haemophilus influenzae (including β-lactamase-negative ampicillin-resistant strains), and Neisseria gonorrhoeae (including ciprofloxacin-resistant strains), with MIC(90)s of ≤ 1 μg/ml. Unlike tebipenem (MIC(50), 8 μg/ml), SM-295291 and SM-369926 had no activity against hospital pathogens such as Pseudomonas aeruginosa (MIC(50), ≥ 128 μg/ml). The bactericidal activities of SM-295291 and SM-369926 against penicillin-resistant S. pneumoniae and β-lactamase-negative ampicillin-resistant H. influenzae were equal or superior to that of tebipenem and greater than that of cefditoren. The therapeutic efficacies of intravenous administrations of SM-295291 and SM-369926 against experimentally induced infections in mice caused by penicillin-resistant S. pneumoniae and β-lactamase-negative ampicillin-resistant H. influenzae were equal or superior to that of tebipenem and greater than that of cefditoren, respectively, reflecting their in vitro activities. SM-295291 and SM-369926 showed intravenous pharmacokinetics similar to those of meropenem in terms of half-life in monkeys (0.4 h) and were stable against human dehydropeptidase I. SM-368589 and SM-375769, which are medoxomil esters of SM-295291 and SM-369926, respectively, showed good oral bioavailability in rats, dogs, and monkeys (4.2 to 62.3%). Thus, 2-aryl carbapenems are promising candidates that show an ideal broad spectrum for the treatment of community-acquired infections, including infections caused by penicillin-resistant S. pneumoniae and β-lactamase-negative ampicillin-resistant H. influenzae, have low selective pressure on antipseudomonal carbapenem-resistant nosocomial pathogens, and allow parenteral, oral, and switch therapies.
Figures


References
-
- Saga T, Yamaguchi K. 2009. History of antimicrobial agents and resistant bacteria. Jpn. Med. Assoc. J. 52:103–108
-
- Livermore DM, Carter MW, Bagel S, Wiedemann B, Baquero F, Loza E, Endtz HP, van Den Braak N, Fernandes CJ, Fernandes L, Frimodt-Moller N, Rasmussen LS, Giamarellou H, Giamarellos-Bourboulis E, Jarlier V, Nguyen J, Nord CE, Struelens MJ, Nonhoff C, Turnidge J, Bell J, Zbinden R, Pfister S, Mixson L, Shungu DL. 2001. In vitro activities of ertapenem (MK-0826) against recent clinical bacteria collected in Europe and Australia. Antimicrob. Agents Chemother. 45:1860–1867 - PMC - PubMed
-
- Daigle DM, Xerri L. 2008. Susceptibility of Pseudomonas aeruginosa to imipenem is unaffected by exposure to PZ-601 (SMP-601), poster C1-1064. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother., Washington, DC, 25 to 28 October 2008 American Society for Microbiology, Washington, DC
-
- Livermore DM, Mushtaq S, Warner M. 2005. Selectivity of ertapenem for Pseudomonas aeruginosa mutants cross-resistant to other carbapenems. J. Antimicrob. Chemother. 55:306–311 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

