The novel quantitative trait locus GL3.1 controls rice grain size and yield by regulating Cyclin-T1;3
- PMID: 23147796
- PMCID: PMC3515756
- DOI: 10.1038/cr.2012.151
The novel quantitative trait locus GL3.1 controls rice grain size and yield by regulating Cyclin-T1;3
Abstract
Increased crop yields are required to support rapid population growth worldwide. Grain weight is a key component of rice yield, but the underlying molecular mechanisms that control it remain elusive. Here, we report the cloning and characterization of a new quantitative trait locus (QTL) for the control of rice grain length, weight and yield. This locus, GL3.1, encodes a protein phosphatase kelch (PPKL) family - Ser/Thr phosphatase. GL3.1 is a member of the large grain WY3 variety, which is associated with weaker dephosphorylation activity than the small grain FAZ1 variety. GL3.1-WY3 influences protein phosphorylation in the spikelet to accelerate cell division, thereby resulting in longer grains and higher yields. Further studies have shown that GL3.1 directly dephosphorylates its substrate, Cyclin-T1;3, which has only been rarely studied in plants. The downregulation of Cyclin-T1;3 in rice resulted in a shorter grain, which indicates a novel function for Cyclin-T in cell cycle regulation. Our findings suggest a new mechanism for the regulation of grain size and yield that is driven through a novel phosphatase-mediated process that affects the phosphorylation of Cyclin-T1;3 during cell cycle progression, and thus provide new insight into the mechanisms underlying crop seed development. We bred a new variety containing the natural GL3.1 allele that demonstrated increased grain yield, which indicates that GL3.1 is a powerful tool for breeding high-yield crops.
Figures

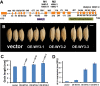
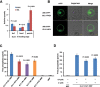
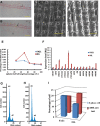


References
-
- Tilman D, Fargione J, Wolff B, et al. Forecasting agriculturally driven global environmental change. Science. 292:281–284. - PubMed
-
- Hibberd JM, Sheehy JE, Langdale JA. Using C4 photosynthesis to increase the yield of rice-rationale and feasibility. Curr Opin Plant Biol. 2008;11:228–231. - PubMed
-
- Khush GS. Green revolution: preparing for the 21st century. Genome. 1999;42:646–655. - PubMed
-
- Khush GS. What it will take to feed 5.0 billion rice consumers in 2030. Plant Mol Biol. 2005;59:1–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

