Timing of the introduction of complementary foods in infancy: a randomized controlled trial
- PMID: 23147979
- PMCID: PMC9923596
- DOI: 10.1542/peds.2011-3838
Timing of the introduction of complementary foods in infancy: a randomized controlled trial
Abstract
Objective: To increase knowledge on iron status and growth during the first 6 months of life. We hypothesized that iron status would be better in infants who received complementary foods in addition to breast milk compared with those exclusively breastfed.
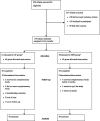
Methods: One hundred nineteen healthy term (≥37 weeks) singleton infants were randomly assigned to receive either complementary foods in addition to breast milk from age 4 months (CF) or to exclusive breastfeeding for 6 months (EBF). Dietary data were collected by 3-day weighed food records, and data on iron status and growth were also collected.
Results: One hundred infants (84%) completed the trial. Infants in the CF group had higher mean serum ferritin levels at 6 months (P = .02), which remained significant when adjusted for baseline characteristics. No difference was seen between groups in iron deficiency anemia, iron deficiency, or iron depletion. The average daily energy intake from complementary foods of 5-month-olds in the CF group was 36.8 kJ per kg body weight. Infants in both groups grew at the same rate between 4 and 6 months of age.
Conclusions: In a high-income country, adding a small amount of complementary food in addition to breast milk to infants' diets from 4 months of age does not affect growth rate between 4 and 6 months, but has a small and positive effect on iron status at 6 months. The biological importance of this finding remains to be determined.
Conflict of interest statement
Figures

References
-
- World Health Organization. The Optimal Duration of Exclusive Breastfeeding: Report of an Expert Consultation (WHO/NHD/01.09). Geneva, Switzerland: Department of Nutrition for Health and Development and Department of Child and Adolescent Health and Development, World Health Organization, 2001
-
- Kramer MS, Kakuma R. The Optimal Duration of Exclusive Breastfeeding: A Systematic Review (WHO/NHD/01.08). Geneva, Switzerland: Department of Nutrition for Health and Development and Department of Child and Adolescent Health and Development, World Health Organization: 2001
-
- World Health Organization . Nutrition: Information and attitudes among health personnel about early infant-feeding practices. Wkly Epidemiol Rec. 1995;70(17):117–120 - PubMed
-
- Kramer MS , Chalmers B , Hodnett ED , et al. PROBIT Study Group (Promotion of Breastfeeding Intervention Trial) . Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285(4):413–420 - PubMed
-
- Monterrosa EC , Frongillo EA , Vásquez-Garibay EM , Romero-Velarde E , Casey LM , Willows ND . Predominant breast-feeding from birth to six months is associated with fewer gastrointestinal infections and increased risk for iron deficiency among infants. J Nutr. 2008;138(8):1499–1504 - PubMed

