Metabolically competent human skin models: activation and genotoxicity of benzo[a]pyrene
- PMID: 23148024
- PMCID: PMC3551429
- DOI: 10.1093/toxsci/kfs316
Metabolically competent human skin models: activation and genotoxicity of benzo[a]pyrene
Abstract
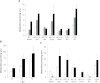
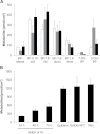
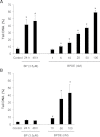
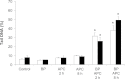
The polycyclic aromatic hydrocarbon (PAH) benzo[a]pyrene (BP) is metabolized into a complex pattern of BP derivatives, among which the ultimate carcinogen (+)-anti-BP-7,8-diol-9,10-epoxide (BPDE) is formed to certain extents. Skin is frequently in contact with PAHs and data on the metabolic capacity of skin tissue toward these compounds are inconclusive. We compared BP metabolism in excised human skin, commercially available in vitro 3D skin models and primary 2D skin cell cultures, and analyzed the metabolically catalyzed occurrence of seven different BP follow-up products by means of liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). All models investigated were competent to metabolize BP, and the metabolic profiles generated by ex vivo human skin and skin models were remarkably similar. Furthermore, the genotoxicity of BP and its derivatives was monitored in these models via comet assays. In a full-thickness skin, equivalent BP-mediated genotoxic stress was generated via keratinocytes. Cultured primary keratinocytes revealed a level of genotoxicity comparable with that of direct exposure to 50-100 nM of BPDE. Our data demonstrate that the metabolic capacity of human skin ex vivo, as well as organotypic human 3D skin models toward BP, is sufficient to cause significant genotoxic stress and thus cutaneous bioactivation may potentially contribute to mutations that ultimately lead to skin cancer.
Figures

References
-
- Boffetta P., Jourenkova N., Gustavsson P. (1997). Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control 8, 444–472 - PubMed
-
- Bosetti C., Boffetta P., La Vecchia C. (2007). Occupational exposures to polycyclic aromatic hydrocarbons, and respiratory and urinary tract cancers: A quantitative review to 2005. Ann. Oncol. 18, 431–446 - PubMed
-
- Cavalieri E. L., Rogan E. G. (1992). The approach to understanding aromatic hydrocarbon carcinogenesis. The central role of radical cations in metabolic activation. Pharmacol. Ther. 55, 183–199 - PubMed
-
- Cavalieri E. L., Rogan E. G. (1995). Central role of radical cations in metabolic activation of polycyclic aromatic hydrocarbons. Xenobiotica 25, 677–688 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

